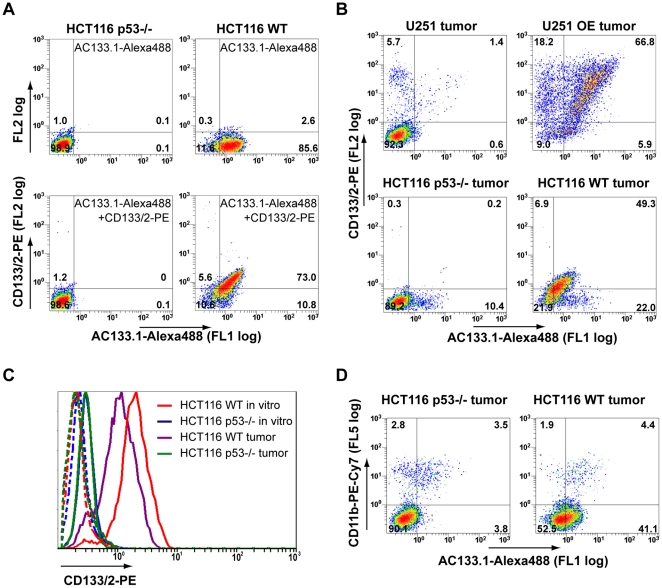
Figure 4. Flow cytometric detection of i.v.-injected AC133.1-Alexa488 antibody on cells isolated from tumors.
(A) Pre-test for tumor digestion experiments showing that CD133 and prebound antibody can be detected after blendzyme/accutase digestion. HCT116 wild-type and HCT116 p53−/− cells were incubated with AC133.1-Alexa488 overnight and digested with blendzyme/accutase for 50 min. The treated cells were then stained with CD133/2-PE antibody and analyzed by flow cytometry. (B) Blendzyme/accutase digested tumors of AC133-Alexa488 injected mice were stained with CD133/2-PE antibody and analyzed by flow cytometry. (C) Downregulation of CD133 by s.c. growing HCT116 wild-type cells. Cells from digested tumors and in vitro cultured HCT116 cells were stained with CD133/2-PE antibody and CD133 expression levels were determined by flow cytometry. The dotted lines show the unstained controls. (D) Tumor macrophages contribute to the signal of in vivo injected AC133-Alexa488. Digested tumors were incubated with anti-mouse Fc-receptor blocking antibodies, stained thereafter with PE-Cy7-CD11b antibody and analyzed by flow cytometry. In Figures 4A, B, and D, the percentages of cells falling into the negative, single- and double-positive quadrants are shown. The quadrants were set according to the autofluorescence of the cells (for the gating controls and for statistical analyses, see Figures S2, S3, S4, and S5). Due to the lower CD133 expression and lower antibody binding in vivo compared to in vitro, the percentage of the cells in the different quadrants is difficult to precisely determine for HCT116 wild-type tumors but the shift towards the double-positive quadrant of the majority of cells can clearly be seen (lower right panel in B; see also Figure S4). FL, fluorescence; WT, wild-type; OE, overexpressing.