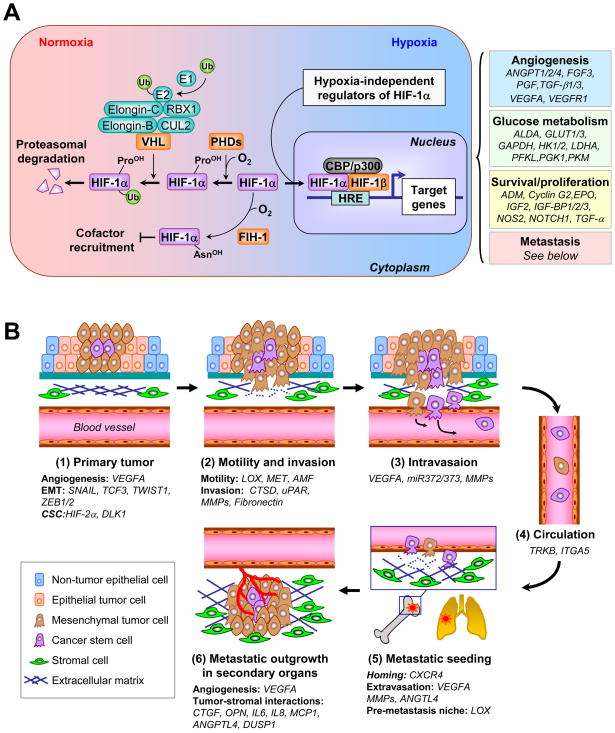
Figure 1. Hypoxia regulation of the metastasis cascade.
(A) In the presence of oxygen (O2), PHDs hydroxylate proline residues of HIF-1α, a reaction that can be inhibited by iron chelation. Hydroxylated HIF-1α interacts with VHL, which is part of an E3 ubiquitin ligase complex that mediates the ubiquitination (Ub) of HIF-1α and targets HIF-1α for degradation in the proteasome. Under hypoxia, HIF-1α is not hydroxylated and is released from VHL-mediated degradation. Stabilized HIF-1 moves into the nucleus, dimerizes with HIF-1β and binds to the hypoxia response element (HRE). By interacting with cofactors such as CBP/p300 and DNA polymerase II, HIF-1 activates transcription of target genes. Binding to CBP/p300 is blocked when HIF-1α is hydroxylated by FIH-1. HIF-1α is additionally regulated by oncogenic pathways (e.g., ERBB2, SRC, ET-1, RAS/MARK pathway, PI3K-Akt-mTOR pathway), mutations of tumor suppressor genes (e.g., PTEN, VHL, SDH and FH), and reactive oxygen species (ROS). HIF-1 target genes related to cancer are categorized into four groups, with representative genes in each group listed. (B) The role of hypoxia responsive genes in different steps of metastasis. 1) The primary response to hypoxia during primary tumor growth is the induction of angiogenesis through VEGFA. HIFs also enhance cancer stem cell self-renewal ability partly through DLK1. Hypoxia activates the expression of SNAIL, TWIST1, TCF3, ZEB1 and ZEB2 to promote epithelial-mesenchymal transition of tumor cells. 2) Tumor cells acquire additional motility under hypoxia with increased expression of LOX, MET and AMF. Furthermore, hypoxia promotes invasion through basement membrane (BM) and extracellular matrix (ECM) by upregulating proteases such as CTSD, uPAR and MMPs. Increased production of fibronectin by hypoxia facilitates the establishment of a tumor-specific ECM. 3) Hypoxia facilitates intravasation by increasing the expression of VEGFA, miR-372/373 and MMPs. 4) Circulating tumor cells may acquire the resistance to anoikis by activation of TrkB and repression of integrin α5 (ITGA5), both of which are hypoxia targets. 5) After reaching the secondary vasculature, tumor cells extravasate using hypoxia-induced molecules such as ANGPTL4, MMPs and VEGFA. Hypoxia-dependent induction of CXCR4 and LOX mediates tumor cell homing to secondary organs and formation of pre-metastatic niche, respectively. 6) In the parenchyma of the distant organ, hypoxia response factors such as ANGPTL4, CXCR4, DUSP1, CTGF, OPN, IL6, IL8 and MCP1 facilitate tumor-stromal interactions to foster the formation of overt metastasis in distinct organs.