Abstract
Background
Hypoglycemia and hyperglycemia can pose a number of serious risks to pregnant mothers with diabetes, but these risks are not always related to glucose concentrations directly. Previous studies have shown the utility of using mathematical transformation functions to create patient risk profiles that can then be used to analyze and predict adverse outcomes in individuals with diabetes. We propose a novel use of these functions to analyze the risks posed to the fetus in pregnancies complicated by diabetes.
Methods
We retrospectively analyzed 71 h continuous glucose monitoring system (CGMS Gold, Medtronic Northridge, CA) third trimester tracings obtained during a normal pregnancy and in those complicated by gestational diabetes mellitus (GDM), type 2 diabetes mellitus (T2DM), and type 1 diabetes mellitus (T1DM). We then used a transformation function to calculate fetal and maternal risk in each case.
Results
In the normal pregnancy (0.93), the risk was at a minimum. Along with mean glucose values, the risk increased in those cases where gestation was complicated by GDM (3.12), T2DM (7.85), and T1DM (16.94). In contrast, the original patient risk profile yielded a minimal value for the GDM tracings.
Conclusions
Total fetal risk increases from normal to GDM to T2DM to T1DM pregnancies. This new risk assignment better distinguishes the stages of fetal risk than the original method and therefore may be useful in future clinical trials and applications to predict risk for adverse outcomes in pregnancies complicated by diabetes.
Keywords: continuous glucose monitoring, fetal risk, gestational diabetes mellitus, high blood glucose index, low blood glucose index, transform function
Introduction
In pregnancies complicated by diabetes, glycemic excursions can seriously compromise the health of both the mother and the fetus. As blood glucose (BG) levels approach the hypoglycemic range, the health risks can include disruptions in cognitive function, loss of consciousness, coma, and even death.1 The maternal risks due to hyperglycemia include hypertension, vascular complications such as retinopathy and nephropathy, organ damage, and other metabolic problems such as diabetic ketoacidosis, a life-threatening condition most commonly observed in women with type 1 diabetes (T1DM).2 For women with gestational diabetes mellitus (GDM), many of these diabetes-related complications will naturally resolve upon delivery; however, these individuals may be in jeopardy of developing diabetes in the future.3,4
During the periconceptional time frame and the first trimester, the risks to the fetus due to hyperglycemia include spontaneous abortion and congenital malforma-tions.5,6 Health risks in the second and third trimesters include macrosomia, birth trauma, premature labor, abnormal fetal growth, and even fetal demise. Macro-somia can itself lead to a number of fetal maladies, including shoulder dystocia, Erb’s palsy, obesity, and impaired neurological development. Children of mothers with diabetes also have an increased risk of developing diabetes themselves.7,8 Hypoglycemia, however, is not usually associated with adverse fetal outcomes.9
Previous studies have attempted to quantify the health risks posed to nonpregnant individuals with diabetes through the use of mathematical transform functions.11–13 Kovatchev et al.14 proposed a logarithmic function that transforms the skewed BG distribution into a symmetric data set. The transformation utilizes parameters based on the 1993 Diabetes Control and Complications Trial report and takes into account nearly all observed glucose levels (20–600 mg/dl or 1.1–33.3 mmol/liter).15 According to the standard BG range, the numerical center is 310 mg/dl or 17.2 mmol/liter. As one can see, the ranges are clearly not symmetrical given that the hypoglycemic range (BG <70 mg/dl or <3.9 mmol/liter) represents only a small portion of the possible values. Many statistical tests are based on the assumption of a symmetric distribution. The skewed nature of the glucose level distribution violates this assumption and renders many of these statistical tests unusable, as the differences in the high and low BG scales present in innate bias. With the logarithmic function, however, the center of the transformed BG range is 112.5 mg/dl or 6.25 mmol/liter, which is clinically accurate, as it represents the midpoint of the euglycemic range.13,15
The resulting plot can be interpreted as the amount of risk associated with a particular BG level.13 The raw data are transformed into a symmetric parabolic function with a theoretical minimum risk value of 0 and a theoretical maximum risk value of 100 to correlate with the extreme BG levels that have been clinically observed. The target euglycemic BG level of 112.5 mg/dl (6.25 mmol/liter) is assigned a risk of 0. The left and right branches of the parabola are defined as low blood glucose index (LBGI) and high blood glucose index (HBGI), which represent the risk levels associated with the hypoglycemia and the hyperglycemia range, respectively. The risk value increases for each as the frequency and/or extent of hypoglycemic and hyperglycemic BG excursions increases. Thus a high risk index may indicate a large number of small glucose deviations, a few instances of large excursions, or a mixture of both.16 The sum of these two nonnegative indices can have an upper limit of 100.
Though glycemic excursions affect the health of both mother and fetus, the risk to each is different. Given the differences between fetal and maternal physiologies, previous transform functions may fail to detect significant risks to the fetus. As such, we propose a new transform function that can accurately assess the health risks to the fetus in pregnancies complicated by diabetes.
Methods
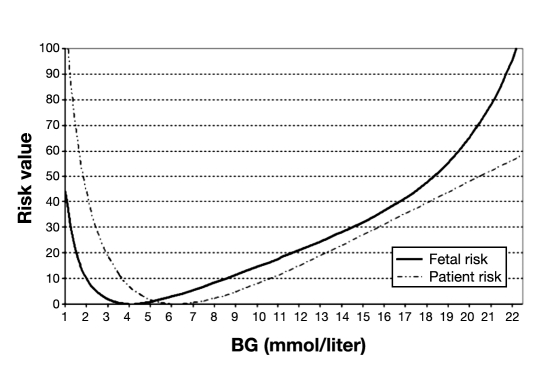
We adapted the original patient risk function described by Kovatchev and colleagues into one suitable to better quantify fetal health risks.14 As shown in Figure 1, the fetal risk curve is shifted leftward compared to that of the patient risk function. This is because, in pregnancies not complicated by diabetes, normal maternal glucose levels are significantly lower than nonpregnant individuals in the first trimester and may level off or continue to decrease slightly throughout gestation.17 The optimal fetal glycemic range is based on the assumption that target levels in diabetic pregnancies should mimic those in normal pregnancies. Studies have shown that the normal fasting glycemic range in healthy pregnancies is 69–75 mg/dl or 3.83–4.16 mmol/liter.18–20 We assigned a risk of 0 to the midpoint of this target range, which is 72 mg/dl or 4 mmol/liter. As such, the original patient risk curve had to be shifted leftward to match this fetal glycemic target. Thus a healthy fetus naturally develops in a glycemic environment that, from the standpoint of a nonpregnant individual, would be considered hypoglycemic. Hypoglycemia is not usually associated with adverse fetal outcomes,9 therefore the fetal risk ascribed to such glucose levels by the original transform function is actually considerably less. There is, however, a historical report in the literature linking insulin shock therapy during pregnancy with adverse outcomes.10
Figure 1.
Comparison of risk transform functions. The solid curve represents risk to the fetus derived from maternal glucose levels. The dotted curve indicates the risk to the mother derived from the original transform function.
Yet in Figure 1, one will note that there is still an increased risk as fetal glycemic levels descend into the hypo- glycemic range. At this point, the maternal risk of a catastrophic event due to severe hypoglycemia begins to increase dramatically. Though the risk value is calculated from the standpoint of the fetus, hypoglycemic maternal and fetal risk is still somewhat linked. For example, if a severe hypoglycemic event were to occur while the mother was operating a motor vehicle, resulting in a fatal accident, the mother and the fetus would obviously experience the same outcome. Though the fetus may not face the same hypoglycemic health risks as the mother per se, there is still a heightened risk of a catastrophic event; the increased risk on the fetal risk plot accounts for this fact.
In the hyperglycemic range, the risk to the fetus is more pronounced in comparison to that of the mother. For instance, the risk of fetal demise is accelerated around 250 mg/dl (13.8 mmol/liter) where there is a greater danger of diabetic ketoacidosis. Along with fetal demise, a number of other health risks exist, including congenital abnormalities and abnormal growth. As such, the fetal risk plot consistently exceeds the maternal risk profile in the hyperglycemic range as shown in Figure 1.
To test the utility of this new fetal risk transformation, we retrospectively analyzed 71 h continuous glucose monitoring (CGM) tracings (CGMS® GOLD™, Medtronic, Northridge, CA) in normal pregnancies and pregnancies complicated by T1DM, type 2 diabetes (T2DM), and GDM. Though the CGM sensors had a life span of 72 h, some data points at the tail end of the data set were removed to ensure that there were an equal number of CGM readings for each case. The tracings were obtained during the third trimester, and the following fetal transformation procedure was used to convert the data into four representative fetal risk profiles.
Once retrieved from the CGM device, each BG reading was transformed using the formula f(BG)= 1.509 × [(ln (BG ))0.50927 - 2.10008], where BG is measured in millimole per liter. The logarithmic argument may be multiplied by 18 to convert to milligrams per deciliter. We then converted the transformed data into risk values according to the function r(BG)= 10 × f(BG)2. The LBGI and HBGI were calculated by splitting the parabolic transformation function as follows: rl(BG)= r(BG) if f(BG) <0 and 0 otherwise (left branch); rh(BG)= r(BG) if f(BG) >0 and 0 otherwise (right branch). Lastly, the total risk was calculated by taking the average of the risk readings aggregated in a given 1 h set of CGM readings and summing the LBGI and HBGI to find the total risk value for each patient.
Results
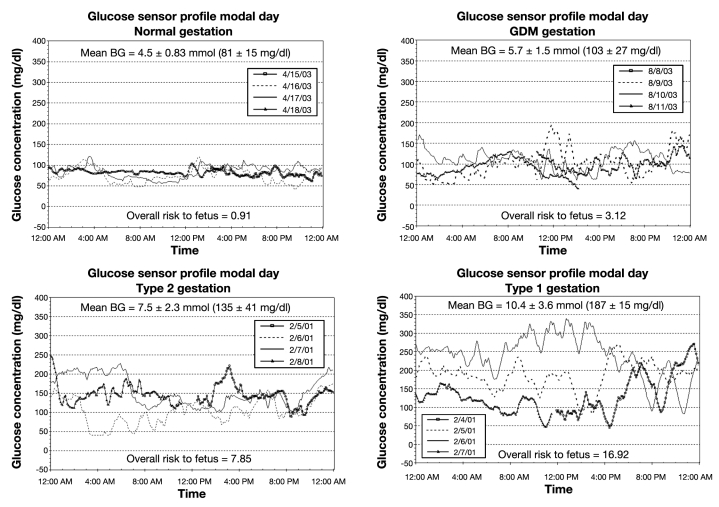
As Figure 2 shows, the fetal risk in the normal pregnancy was minimal at 0.91. Since a risk value of 0 is a theoretical limit, even healthy patients will exhibit some risk. The results show that the risk increased in those cases complicated by GDM, T2DM, and T1DM, which was 3.12, 7.85, and 16.92, respectively (Table 1). As shown in Figure 2, the fetal risk increased in those cases that exhibited poor glycemic control, both in terms of mean glucose and with respect to the frequency and extent of glycemic excursions.
Figure 2.
The resulting fetal risk value along with the mean blood glucose derived from consecutive 71 h CGM tracings.
Table 1.
Fetal Risk for Adverse Outcome Based on Maternal Continuous Glucose Sensinga
| Gestation | Fetal risk | Glucose mg/dl (mean ± standard deviation) |
|---|---|---|
| Normal | 0.91 | 81 ± 15 |
| GDM | 3.12 | 103 ± 27 |
| Type 2 diabetes | 7.85 | 135 ± 41 |
| Type 1 diabetes | 16.92 | 187 ± 15 |
These calculations are based on a single 71 h CGM session per individual.
Discussion
As expected, mean glucose levels and total fetal risk increased from normal to GDM to T2DM to T1DM gestation. The normal pregnancy profile did not exhibit a risk of 0, but of 0.91. This can be explained both by the noise of the CGM electrochemical sensor and by benign variation within the euglycemic target range, of which the transform function assigned a minimal risk value. Of considerable note was the difference between the patient and fetal risk levels in the case of GDM gestation, which suggests that the new transform function can discern fetal risk better than the original maternal risk formula.
Though the original transform expression was developed on an intuitive basis, studies have confirmed that this function can indeed accurately normalize raw BG data.11–13 In addition, studies have shown that the HBGI/LBGI statistics have value in predicting hyperglycemic and hypoglycemic events. In a study by Kovatchev et al.,16 96 patients with T1DM underwent one month of self-monitoring of blood glucose (SMBG) therapy. Participants were then asked to record any severe hypoglycemic events for the next six months. Researchers transformed data stored in the glucose monitors into risk profiles for each patient. Individuals who had a higher LBGI reported having a significantly higher number of hypo-glycemic events than those who had a lower value. Researchers reported that, along with previous history of severe hypoglycemia, LBGI played a significant role in the prediction of these future hypoglycemic events. They also showed that individuals with a high risk value were more likely to have a severe hypoglycemic event occur sooner than those with a lower risk profile.
Risk analysis provides a distinct advantage over traditional evaluative methods such as average BG and glycosylated hemoglobin (A1C) levels.11,13 Glycemic excursions do not meaningfully contribute to changing A1C beyond their effect on mean BG.21 For example, a patient with optimal glucose control and another individual with large, yet opposite glycemic excursions may have a similar A1C, because the mean glucose levels will be the same; however, the former patient will have a lower risk profile. Similar to A1C levels, the skewed distribution of the raw glucose values can also cause average BG measurements to be misleading. Take for example patient 1 who has two glucose measurements of [90, 90 mg/dl] and patient 2who has values of [40, 140 mg/dl]. An average BG measurement would yield 90 mg/dl for each patient, though the former exhibits less risk. The benefit of risk analysis is that it can, through the use of LBGI and HBGI, account for this skewed distribution and predict adverse outcomes equally well in both the hypo- and hyperglycemic ranges.
The risk function is also advantageous in that it attenuates glucose excursions in the target range, which is aligned with the clinical understanding that glucose fluxes in the euglycemic range are relatively benign. Conversely, the risk function assigns a weighted risk to more extreme fluctuations, which corresponds to the notion that such large fluxes carry more health risks. Unlike standard deviation values, risk functions are based on defined parameters and do not represent a relative risk value, but an absolute value that can be compared to other patients.10 The fetal risk transformation formulas are very manageable and can be easily incorporated into spreadsheet software or other user-friendly computer programs. Risk assessment data can then be presented as risk traces, Poincaré plots, and other visual representations that facilitate patient and physician comprehension. Please note that these retrospective tracings represent only a brief snapshot of maternal glycemia during a 9-month-long gestation. Improvements in CGM technology allow for improved sensor accuracy and extended duration of wear. Additionally, one must consider glycemic variability in addition to absolute glucose concentrations when calculating the maternal and fetal risk.
Conclusions
Careful monitoring of BG is essential for proper glycemic control.22 It has been shown that achieving euglycemia can reduce the risk of maternal and fetal morbidities to that of normal pregnancies.6,23 The introduction of CGM technology, with its ability to provide real-time CGM measurements and predict future glycemic excursions, represents a major advancement in glucose monitoring methods and a powerful tool in the fight against diabetes. With CGM systems, clinicians now have access to a consistent and large data set that can be used to assess a patient’s health risk more accurately. Previously used with SMBG, risk analysis and its predictive power may greatly change with CGM devices that can provide much more raw data, which, in turn, may strengthen the power of the statistical tests used.
In the long term, fetal risk analysis, in conjunction with A1C and other analytical methods, may help physicians improve their ability to assess the future health risks of their patients. The prevalence of glycemic variation in the present can give clinicians valuable clues about how their patients will fare in the future. Comparisons of these risk values can also help clinicians assess which patients are in greater need of intervention. As time and resources in clinics and hospitals are limited, fetal risk assessment can be used to stratify patients so that physicians can focus their efforts on individuals who are in dire need of medical care. Though further study is needed to confirm the accuracy of the new risk transform function, fetal risk assessment may prove valuable in future clinical trials and practices in helping clinicians predict the risk for adverse perinatal outcomes in pregnancies complicated by diabetes.
Abbreviations
- (A1C)
glycosylated hemoglobin
- (BG)
blood glucose
- (CGM)
continuous glucose monitoring
- (GDM)
gestational diabetes mellitus
- (HBGI)
high blood glucose index
- (LBGI)
low blood glucose index
- (SMBG)
self-monitoring of blood glucose
- (T1DM)
type 1 diabetes mellitus
- (T2DM)
type 2 diabetes mellitus
References
- 1.The Diabetes Control and Complications Trial Research Group Hypoglycemia in the Diabetes Complications and Control Trial. Diabetes. 1997;46(2):271–286. [PubMed] [Google Scholar]
- 2.American College of Obstetricians and Gynecologists . Clinical management guidelines for obstetrician-gynecologists. Washington DC: American College of Obstetricians and Gynecologists; 2005. ACOG practice bulletin no. 60. [DOI] [PubMed] [Google Scholar]
- 3.American College of Obstetricians and Gynecologists . Clinical management guidelines for obstetrician-gynecologists. Washington DC: American College of Obstetricians and Gynecologists; 2001. ACOG practice bulletin no. 30. [PubMed] [Google Scholar]
- 4.Diabetes Care; Proceedings of the fourth international work-shop-conference on gestational diabetes mellitus; 14-16 March 1997; Chicago, Illinois, USA. 1998. pp. B1–B167. [PubMed] [Google Scholar]
- 5.Mills JL, Simpson JL, Driscoll SG, Jovanovic-Peterson L, Van Allen M, Aarons JH, Metzger B, Bieber FR, Knopp RH, Holmes LB, Peterson C, Withiam-Wilson M, Brown Z, Ober C, Harley E, Macpherson T, Duckles A, Mueller-Heubach E. Incidence of spontaneous abortion among normal women and insulin-dependent diabetic women whose pregnancies were identified within 21 days of conception. N Engl J Med. 1988;319(25):1617–1623. doi: 10.1056/NEJM198812223192501. [DOI] [PubMed] [Google Scholar]
- 6.Casson IF, Clarke CA, Howard CV, McKendrick O, Pennycook S, Pharoah PO, Platt MJ, Stranisstreet M, van Velszen D, Walkinshaw S. Outcomes of pregnancy in insulin dependent diabetic women: results of a five year population cohort study. BMJ. 1997;315(7103):275–278. doi: 10.1136/bmj.315.7103.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kitzmiller J, Block M. Glycemic control and perinatal outcome. In: Kitzmiller J, Jovanovic L, Brown F, Coustan D, Reader D, editors. Managing preexisting diabetes and pregnancy: technical reviews and consensus recommendations for care. New York: McGraw-Hill; 2008. [Google Scholar]
- 8.Fetita LS, Sobngwi E, Serradas P, Calvo F, Gautier JF. Consequences of fetal exposure to maternal diabetes in offspring. J Clin Endocrinol Metab. 2006;91(10):3714–3724. doi: 10.1210/jc.2006-0624. [DOI] [PubMed] [Google Scholar]
- 9.Rosenn BM, Miodovnik M, Holcberg G, Khoury JC, Siddiqi TA. Hypoglycemia: the price of intensive insulin therapy for pregnant women with insulin-dependent diabetes mellitus. Obstet Gynecol. 1995;85(3):417–422. doi: 10.1016/0029-7844(94)00415-A. [DOI] [PubMed] [Google Scholar]
- 10.Impastato DJ, Gabriel AR, Lardaro HH. Electric and insulin shock therapy during pregnancy. Dis Nerv Syst. 1964;25:542–546. [PubMed] [Google Scholar]
- 11.Kovatchev BP, Otto E, Cox D, Gonder-Frederick L, Clarke W. Evaluation of a new measure of blood glucose variability in diabetes. Diabetes Care. 2006;29(11):2433–2438. doi: 10.2337/dc06-1085. [DOI] [PubMed] [Google Scholar]
- 12.Kovatchev BP, Cox DJ, Kumar A, Gonder-Frederick L, Clarke WL. Algorithmic evaluation of metabolic control and risk of severe hypoglycemia in type 1 and type 2 diabetes using self-monitoring blood glucose data. Diabetes Technol Ther. 2003;5(5):817–828. doi: 10.1089/152091503322527021. [DOI] [PubMed] [Google Scholar]
- 13.Kovatchev BP, Straume M, Cox DJ, Farhi LS. Risk analysis of blood glucose data: A quantitative approach to optimizing the control of insulin dependent diabetes. J Theor Med. 2000;3:1–10. [Google Scholar]
- 14.Kovatchev BP, Cox DJ, Gonder-Frederick LA, Clarke W. Symmetrization of the blood glucose measurement scale and its applications. (Abstract) Diabetes Care. 1997;20(11):1655–1658. doi: 10.2337/diacare.20.11.1655. [DOI] [PubMed] [Google Scholar]
- 15.Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications of insulin-dependent diabetes mellitus. N Eng J Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 16.Kovatchev BP, Cox DJ, Gonder-Frederick LA, Young-Hyman D, Schlundt D, Clarke W. Assessment of risk for severe hypoglycemia among adults with IDDM: validation of the low blood glucose index. Diabetes Care. 1998;21(11):1870–1875. doi: 10.2337/diacare.21.11.1870. [DOI] [PubMed] [Google Scholar]
- 17.Mills JL, Jovanovic L, Knopp R, Aarons J, Conley M, Park E, Lee YJ, Holmes L, Simpson JL, Metzger B. Physiological reduction in fasting plasma glucose concentration in the first trimester of normal pregnancy: the Diabetes in Early Pregnancy Study. Metabolism. 1998;47(9):1140–1144. doi: 10.1016/s0026-0495(98)90290-6. [DOI] [PubMed] [Google Scholar]
- 18.Campbell DM, Bewsher PD, Davidson JM, Sutherland HW. Day-to-day variations in fasting plasma glucose and fasting plasma insulin levels in late normal pregnancy. J Obstet Gynaecol Br Commonw. 1974;81(8):615–621. doi: 10.1111/j.1471-0528.1974.tb00527.x. [DOI] [PubMed] [Google Scholar]
- 19.Yogev Y, Ben-Haroush A, Chen R, Rosenn B, Hod M, Langer O. Diurnal glycemic profile in obese and normal weight nondiabetic pregnant women. Am J Obstet Gynecol. 2004;191(3):949–953. doi: 10.1016/j.ajog.2004.06.059. [DOI] [PubMed] [Google Scholar]
- 20.Parretti E, Mecacci F, Papini M, Cioni R, Carignani L, Mignosa M, La Torre P, Mello G. Third-trimester maternal glucose levels from diurnal profiles in nondiabetic pregnancies: correlation with sonographic parameters of fetal growth. Diabetes Care. 2001;24(8):1319–1323. doi: 10.2337/diacare.24.8.1319. [DOI] [PubMed] [Google Scholar]
- 21.Derr R, Garrett E, Stacy GA, Saudek CD. Is HbA1c affected by glycemic instability? Diabetes Care. 2003;26(10):2728–2733. doi: 10.2337/diacare.26.10.2728. [DOI] [PubMed] [Google Scholar]
- 22.American Diabetes Association Clinical practice recommendations 2001: standards of medical care for patients with diabetes mellitus. Diabetes Care. 2001;24:S33–S44. [Google Scholar]
- 23.Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS, Australian Carbohydrate Intolerance Study in Pregnant Women (ACHOIS) Trial Group Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005;352(24):2477–2486. doi: 10.1056/NEJMoa042973. [DOI] [PubMed] [Google Scholar]