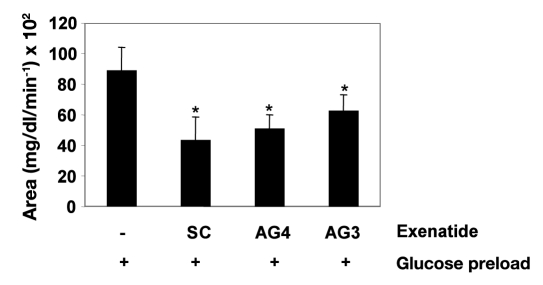
Figure 3.
Pharmacodynamic evaluation of ORMD-0901 formulations in glucose-challenged dogs: mean AUC0–150 of glucose responses of healthy, cannulated dogs to ORMD-0901. Dogs were treated with ORMD-0901 formulation AG3 (n = 8) or AG4 (n = 12), containing 75 or 100 µg exenatide, respectively, 30 min prior to an oral glucose challenge of 80 ml of a 50% dextrose solution + 100 g commercial dog food (7% protein, 6% fat, 1.2% fiber, 80% moisture). For controls, glucose challenge in the absence of exenatide (n = 16) or glucose challenge after subcutaneous injection of 2.5 µg exenatide (n = 10) was performed. Blood was drawn every 15 min from start of experiment, and blood glucose levels were determined using a glucometer. A single asterisk represents p = .068. SC, subcutaneous.