Abstract
The optimal blood pressure (BP) to prevent major adverse outcomes (death, myocardial infarction, and stroke) for patients with hypertension and coronary artery disease who have undergone previous revascularization is unknown but might be influenced by the type of revascularization procedure. We analyzed data from the INternational VErapamil SR-Trandolapril STudy, focusing on the relation between BP and the outcomes of 6,166 previously revascularized patients, using the 16,410 nonrevascularized patients as a reference group. The previous revascularization strategy consisted of coronary artery bypass grafting (CABG, 45.2%), percutaneous coronary intervention (PCI, 42.1%), or both (CABG+PCI, 12.8%). Patients who had undergone both CABG+PCI and CABG-only had a greater adverse outcome risk (adjusted hazard ratio 1.27% and 1.20%, 95% confidence interval 1.06 to 1.53 and 1.07 to 1.35, respectively). The risk was similar for PCI-only patients (adjusted hazard ratio 1.04, 95% confidence interval 0.92 to 1.19). The relations between the adjusted hazard ratio and on-treatment BP appeared J-shaped for each revascularization strategy, accentuated for PCI and diastolic BP (DBP), but excepting CABG only and DBP for which the relation was linear and positive. In conclusion, major adverse outcomes were more frequent in patients with coronary artery disease who had undergone previous CABG, with or without PCI, compared to those with previous PCI only. This likely reflected more severe vascular disease. The relation to systolic BP was J-shaped for each strategy. Among those patients with previous CABG only, the linear relation with DBP suggested that more complete revascularization might attenuate hypoperfusion at a low DBP. The management of BP might, therefore, require modification of targets according to the revascularization strategy to improve outcomes.
The number of patients with coronary artery disease and previous revascularization is increasing, and most of these patients have hypertension.1 Major adverse outcomes (death, myocardial infarction, and stroke) occur more frequently in this group than in similar patients without previous revascularization, in part because of more adverse risk conditions, leading to more severe vascular disease. Additionally, major adverse outcomes have been reported to be related to blood pressure (BP) in a quadratic or J-shape.1 However, the relation between adverse outcomes and BP as a function of a specific previous revascularization strategy (coronary artery bypass grafting [CABG], percutaneous coronary intervention [PCI], or both [CABG+PCI]) in hypertensive patients is unknown. The INternational VErapamil SR-Trandolapril STudy (INVEST)2,3 provides an opportunity to initiate an understanding of this relation. We report the results of a substudy analysis focusing on the 6,166 of 22,576 INVEST patients who had undergone previous revascularization, stratified by the revascularization strategy: CABG-only, PCI-only, or CABG+PCI.
Methods
The INVEST design, methods, and principal results have been previously described in detail (registration site www.clinicaltrials.gov/ct2/show/NCT00133692; registration number NCT00133692).2,3 The study was performed in accordance with the Declaration of Helsinki and approved by the local ethics committees, and all patients provided informed consent. In brief, patients with clinically stable coronary artery disease aged ≥50 years with hypertension were randomized to either a verapamil sustained release (SR)- or an atenolol-based antihypertensive treatment strategy. The addition of trandolapril with or without hydrochlorothiazide was recommended if needed for BP control. Trandolapril was also recommended for patients with heart failure, diabetes, or renal insufficiency. The BP treatment goal was <140/90 mm Hg (or <130/85 mm Hg for patients with diabetes or renal insufficiency). The primary adverse outcome was the first occurrence of all-cause death, nonfatal myocardial infarction, or nonfatal stroke. The secondary outcomes were death, fatal and nonfatal myocardial infarction, fatal and nonfatal stroke, and revascularization. The main results of the INVEST were that both strategies provided excellent BP control (>70% patients achieved <140/90 mm Hg) and were equivalent for reducing mortality and major morbidity.
The original analysis focusing on patients with previous coronary revascularization was prespecified1; however, the present subgroup analysis was not. Patients undergoing revascularization <1 month immediately before enrollment were excluded from the present study. The data on any revascularization procedures ≥1 month before enrollment were collected at baseline, including the specific strategy (CABG, PCI, and CABG+PCI).
The baseline data for each revascularization strategy have been summarized as the mean ± SD for continuous variables and the number and percentage for categorical variables. The pulse pressure was calculated as the difference between the systolic blood pressure (SBP) and diastolic blood pressure (DBP). In the present exploratory analysis, the patients were grouped by 10-mm Hg strata of the average follow-up SBP. The distribution of the primary outcome rate stratified by follow-up BP was evaluated to determine whether the relation was linear. Because the frequency distributions were most commonly consistent with a quadratic function, a quadratic stepwise Cox proportional hazard model was formed for the time interval to the primary outcome for each BP variable (factors for BP and BP2). A similar analysis was conducted for DBP. A SBP of 140 mm Hg and a DBP of 90 mm Hg were used as the reference (hazard ratio [HR] 1.0) within each subgroup.
Kaplan-Meier analysis was used to assess the time interval to the first event for the primary outcome. A stepwise Cox proportional hazard model was used to identify the factors associated with the primary outcome among the 3 revascularization strategy groups and the nonrevascularized reference group (n = 16,410). The following covariates were forced into the model: medical treatment strategy (verapamil SR vs atenolol), age (in decades), gender, race, previous myocardial infarction, and previous congestive heart failure. Other factors were retained in the final model if p ≤0.10 was achieved.
To estimate the risk of the primary outcome for the different revascularization strategies, 3 separate Cox proportional hazard models with factors for CABG, PCI, and CABG+PCI were conducted for the whole population (using the nonrevascularized population as the reference): (1) unadjusted model; (2) stepwise model; and (3) stepwise model with the addition of the average follow-up SBP and DBP (linear and quadratic terms).
To control for the nonrandom assignment of patients to each of the 3 specific revascularization strategies (or to no revascularization), we calculated the propensity score for each patient to be in one group or another, adjusting for all demographic and clinical characteristics available for each patient at the baseline as explanatory variables.4,5 The propensity score was then used as a variable in the Cox proportional hazard model to adjust for any group membership differences attributable to the variables used to create the propensity score. This analysis was performed as a sensitivity analysis to assess whether the difference in baseline characteristics for each group explained the difference in the risk of the primary outcome.
Data management and statistical analyses were performed using Statistical Analysis System statistical software, version 8.2 (SAS Institute, Cary, North Carolina). Statistical significance was assumed when p <0.05 (2-tailed).
Results
Of the 22,576 patients enrolled in INVEST, 6,166 (27.3%) had undergone previous coronary revascularization: CABG, 2,784 (45.2%); PCI, 2,594 (42.1%); and CABG+PCI, 788 (12.8%). Those with CABG+PCI had the greatest prevalence of adverse conditions associated with a high risk of adverse outcomes, including a history of myocardial infarction, congestive heart failure, stroke/transient ischemic attack, diabetes, dyslipidemia, and cigarette smoking (Table 1). Those who had undergone CABG only had the second greatest prevalence of adverse conditions, and those who had undergone PCI only had the lowest. However, those who had undergone CABG only had the lowest prevalence of angina. The revascularized patients, as a group, compared to the nonrevascularized patients tended to be older, more frequently men, white, and United States residents, with a greater prevalence of characteristics associated with a high risk of adverse outcomes.1
Table 1.
Patient characteristics at enrollment according to revascularization strategy
| Characteristic | CABG (n = 2,784) |
PCI (n = 2,594) |
CABG+PCI (n = 788) |
No Previous Revascularization (n = 16,410) |
p Value† |
|---|---|---|---|---|---|
| Age (years) | 68.3 ± 8.8 | 66.2 ± 9.4 | 67.2 ± 9.2 | 65.6 ± 9.9 | <0.001 |
| Age >70 years | 42.7% | 33.3% | 37.1% | 31.6% | <0.001 |
| Body mass index (kg/m2) | 28.7 ± 5.0 | 29.2 ± 8.1 | 30.0 ± 14.7 | 29.2 ± 6.7 | <0.001 |
| Men | 71.3% | 60.8% | 68.4% | 40.8% | <0.001 |
| United States residency | 82.1% | 78.5% | 90.9% | 73.7% | <0.001 |
| Race/ethnicity | <0.001 | ||||
| White | 74.8% | 70.2% | 78.0% | 39.0% | |
| Black | 8.8% | 9.5% | 8.0% | 15.1% | |
| Hispanic | 14.2% | 18.4% | 11.8% | 43.1% | |
| Asian | 0.9% | 0.8% | 1.5% | 0.6% | |
| Other/multiracial | 1.3% | 1.1% | 0.6% | 2.2% | |
| Myocardial infarction | 47.8% | 51.5% | 55.6% | 25.1% | <0.001 |
| Angina pectoris | 31.7% | 38.3% | 42.4% | 78.2% | <0.001 |
| Unstable angina (≥1 month before enrollment) | 18.8% | 22.5% | 31.5% | 7.5% | <0.001 |
| Stroke/transient ischemic attack | 9.3% | 8.0% | 11.0% | 6.6% | 0.023 |
| Left ventricular hypertrophy | 19.5% | 17.9% | 18.3% | 23.1% | <0.001 |
| Arrhythmia | 8.7% | 7.1% | 10.0% | 6.7% | 0.012 |
| Heart failure class I–III | 7.5% | 4.4% | 9.5% | 5.2% | <0.001 |
| Peripheral vascular disease | 15.9% | 11.0% | 15.6% | 11.3% | <0.001 |
| Cigarette smoking (any history) | 58.7% | 58.4% | 60.2% | 41.6% | <0.001 |
| Diabetes | 34.1% | 27.8% | 35.0% | 27.1% | <0.001 |
| Renal impairment | 3.6% | 1.9% | 3.6% | 1.5% | <0.001 |
| Dyslipidemia | 74.0% | 75.4% | 79.1% | 48.5% | 0.013 |
| Antiplatelet drug therapy | 82.7% | 85.9% | 83.5% | 46.4% | 0.004 |
| Lipid-lowering drug therapy | 60.8% | 61.5% | 66.1% | 27.3% | 0.025 |
Data are presented as mean ± SD or %.
Comparing CABG, PCI, and CABG+PCI subpopulations.
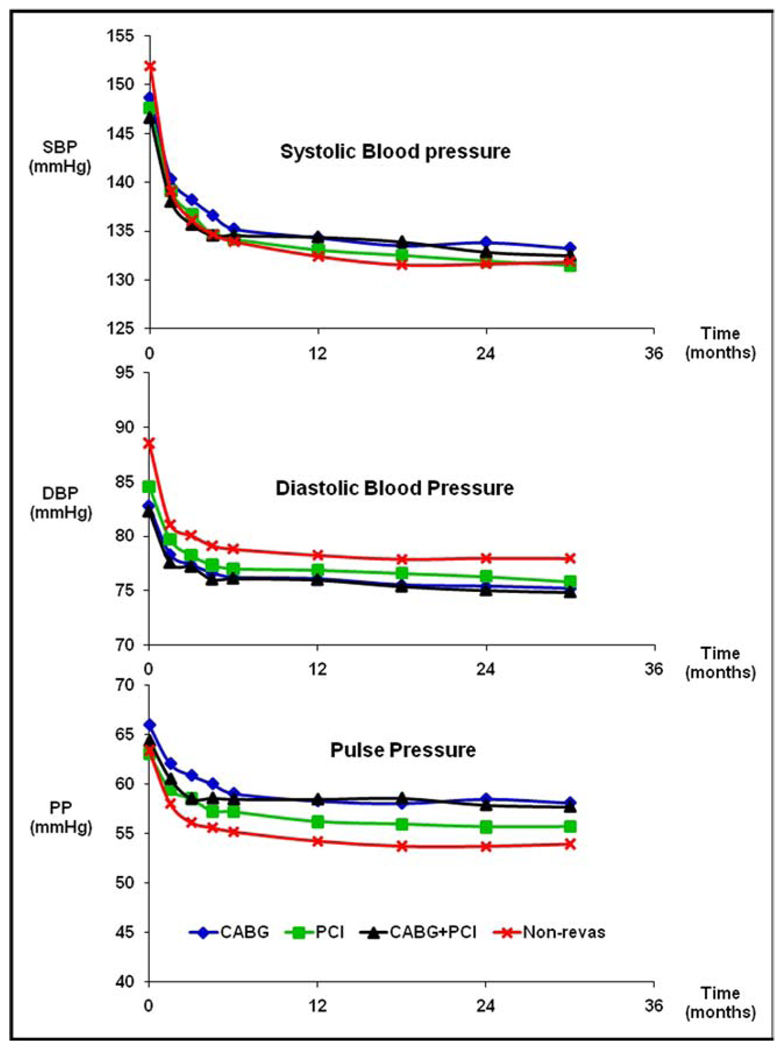
At baseline, the SBP and DBP were similar, regardless of the revascularization strategy used and were lower compared to the SBP and DBP of the nonrevascularized patients (p <0.001 for each revascularization strategy vs no previous revascularization; Figure 1). The pulse pressure was greater for CABG-only patients (p <0.001) but not for the CABG+PCI and PCI-only patients (p >0.05) compared to nonrevascularized patients. The greatest decrease in SBP, DBP, and pulse pressure for all patients occurred during the first 6 weeks of treatment (p <0.001), followed by smaller changes out to 24 months. However, by 24 months, the SBP was uniformly greater and the DBP was uniformly lower for the CABG-only and CABG+PCI patients compared to the PCI-only and nonrevascularized patients (p <0.001). Thus, by 24 months, the pulse pressure was greater for the CABG-only and CABG+PCI patients compared to the PCI-only patients, whose pulse pressure, in turn, was greater than that of the nonrevascularized patients (p <0.001). Among the revascularized patients, the verapamil SR- and atenolol-based treatment strategies resulted in similar control of BP, regardless of the revascularization strategy used (data not shown).
Figure 1.
BP stratified by revascularization strategy and for nonrevascularized patients. Mean follow-up period was 32.9 ± 10.3 months. PP = pulse pressure.
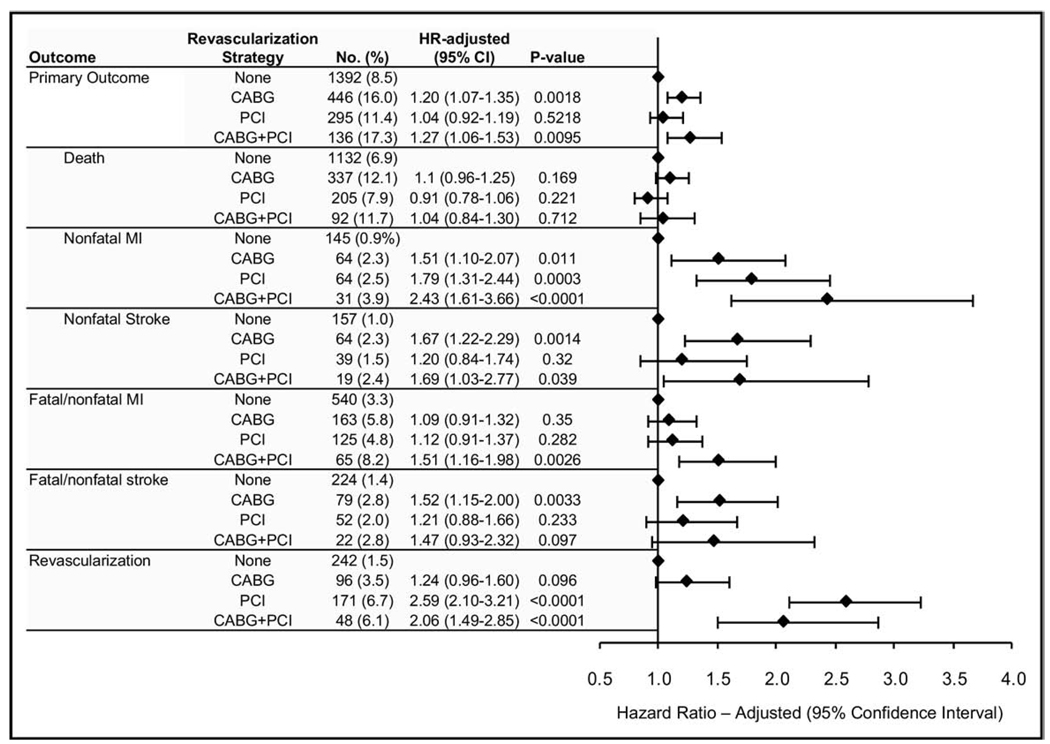
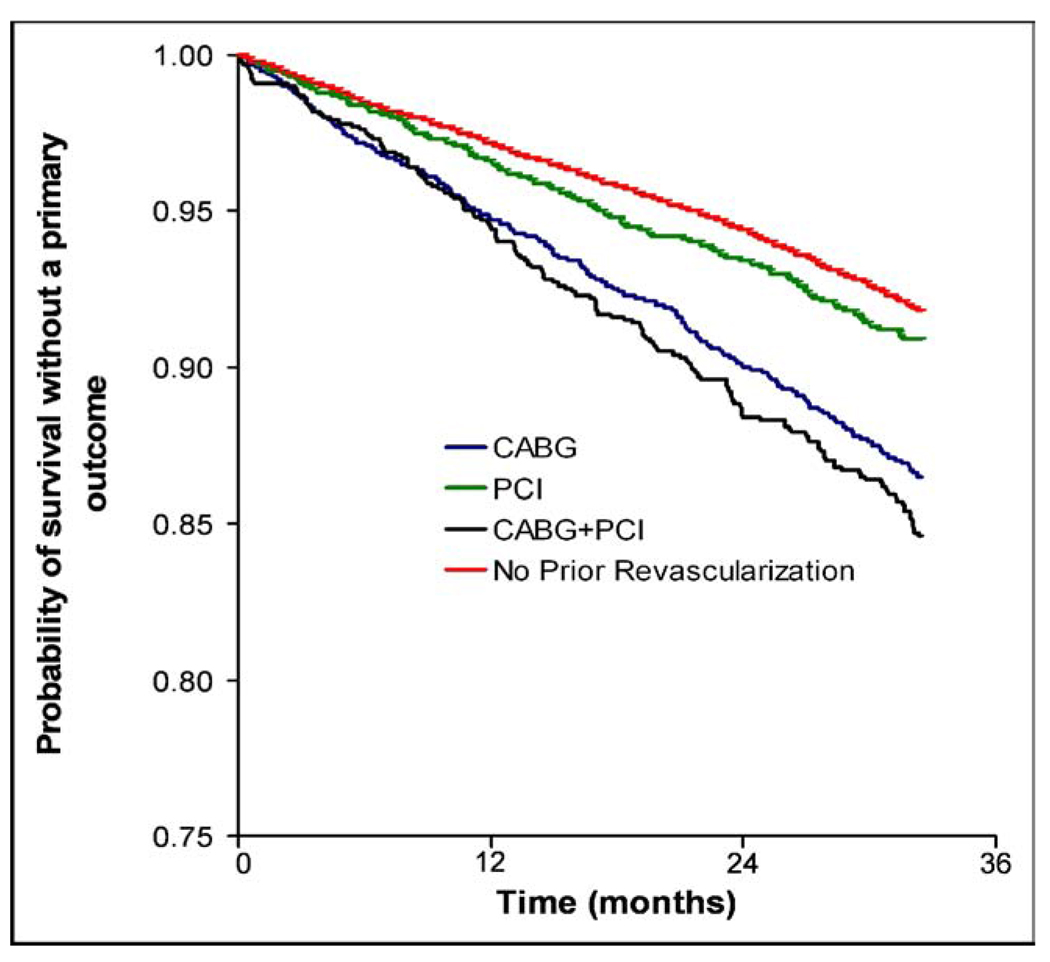
A graded incidence in the primary outcome occurred for the revascularized patients according to the revascularization strategy. The incidence of the primary outcome was greatest for the CABG+PCI patients (136 of 788 [17.3%]), followed by the CABG-only patients (446 of 2,784 [16.0%]), and was lowest for the PCI-only patients (295 of 2,594 [11.4%]; Figure 2). Additionally, the differences between the cumulative primary outcome rates for each strategy increased with time (Figure 3). The unadjusted HR for the primary outcome was also greatest for the CABG+PCI patients, followed by the CABG-only patients, and was lowest for the PCI-only patients (Table 2). Each unadjusted HR was significantly greater than that for the nonrevascularized patients. Additionally, the adjusted and propensity score HRs for the primary outcome for the CABG+PCI and CABG-only patients decreased, but nonetheless remained significantly greater compared to that of the nonrevascularized patients. However, the adjusted and propensity score HRs for the PCI-only patients decreased to a point not significantly different than those of the nonrevascularized patients. The results were similar after adjustment for the baseline conditions and follow-up BP (data not shown).
Figure 2.
Risk of adverse outcomes (adjusted) for revascularized patients according to strategy compared to nonrevascularized patients. MI = myocardial infarction.
Figure 3.
Survival without primary outcome as function of time for revascularized patients stratified by strategy and for nonrevascularized patients.
Table 2.
Hazard ratio (HR) for primary outcome according to revascularization strategy
| Revascularization Strategy | Unadjusted HR (95% CI) | Adjusted HR (95% CI) | Propensity HR (95% CI) |
|---|---|---|---|
| CABG (n = 2,784) | 1.66 (1.50–1.85) | 1.20 (1.07–1.35) | 1.34 (1.00–1.80) |
| PCI (n = 2,594) | 1.16 (1.02–1.31) | 1.04 (0.92–1.19) | 1.06 (0.92–1.21) |
| CABG + PCI (n = 788) | 1.76 (1.48–2.10) | 1.27 (1.06–1.53) | 1.43 (1.03–1.98) |
Unadjusted, adjusted for baseline conditions, and after propensity score adjustment (compared to no previous revascularization).
CI = confidence interval.
The most frequent secondary outcome for all patients was death, followed by (for all patients, except for the PCI-only patients) fatal and nonfatal myocardial infarction (Figure 2). Death occurred most frequently among the CABG-only and CABG+PCI patients, but the incidence was not significantly greater than that for the nonrevascularized patients. However, fatal and nonfatal myocardial infarction occurred most frequently among the CABG+PCI patients and, in contrast to the CABG-only and PCI-only patients, the incidence was significantly greater than that for the nonrevascularized patients. Also, although not as frequent, the incidence of fatal and nonfatal stroke was greatest among the CABG-only and CABG+PCI patients, and the incidence was, at least for the CABG-only patients, significantly greater than that for the nonrevascularized patients. Finally, although revascularization occurred most frequently in the PCI-only and CABG+PCI patients and was significantly greater than that for the nonrevascularized patients, the incidence was still relatively low (6.7% and 6.1%, respectively).
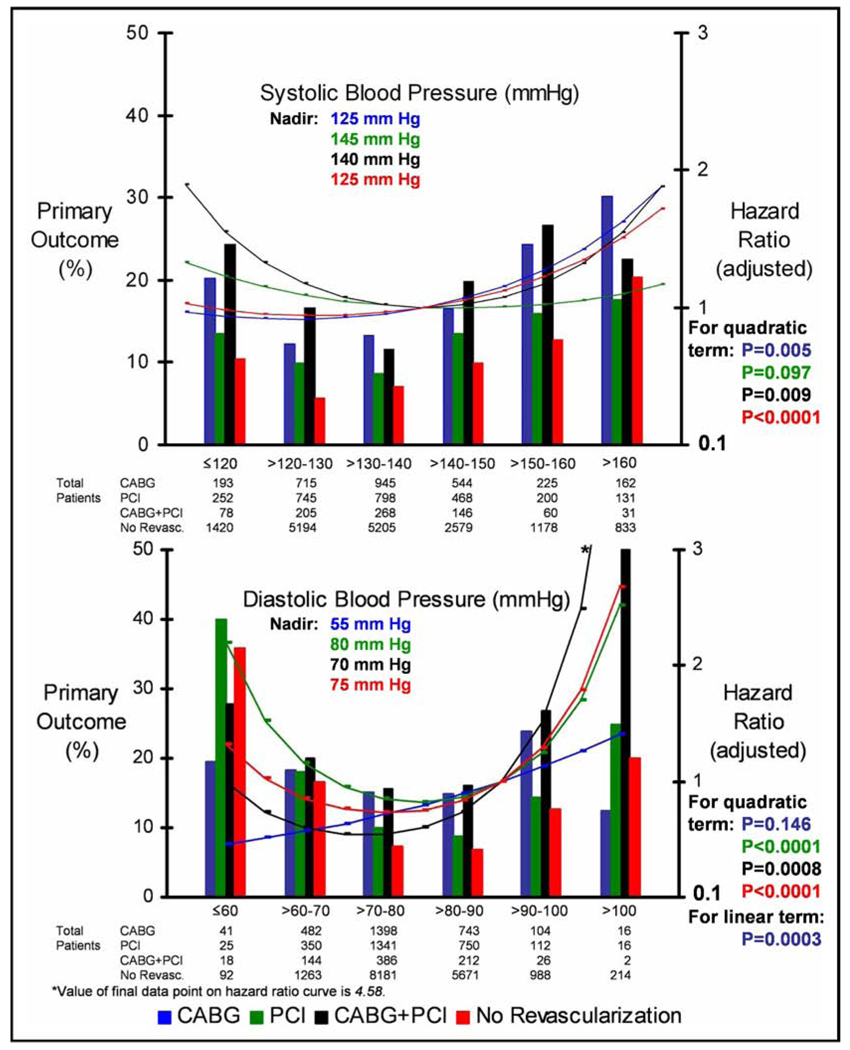
The relations between the incidence of the primary outcome and the mean follow-up SBP and DBP for each of the 3 revascularization strategies were fairly J-shaped (Figure 4). A J-shaped curve was also almost uniformly observed for the relations between the adjusted HR for the primary outcome and the mean follow-up SBP and DBP for these 3 revascularization strategies (Figure 4). This J-shaped curve was accentuated for the PCI patients and DBP. However, for the PCI patients and SBP, the relation was not completely quadratic (p = 0.097 for the quadratic term). Also, for the CABG patients and DBP, the relation was linear and positive (p = 0.0003 for the linear term). Each of these relations persisted after propensity scoring analysis. The SBP/DBP nadir was 125/55 for CABG, 145/80 for PCI, and 140/70 mm Hg for CABG+PCI.
Figure 4.
Incidence of primary outcome and HR (adjusted) as function of SBP and DBP stratified by revascularization strategy. Reference SBP and DBP for HR (adjusted): 140 and 90 mm Hg, respectively.
Discussion
The results of the present INVEST substudy indicate that major adverse outcomes for revascularized patients with previous CABG, with or without PCI, were worse than the outcomes for revascularized patients with previous PCI only, and that outcomes for the PCI-only patients were similar to those of the nonrevascularized patients. Additionally, the results indicate that the relations between the adjusted HRs and follow-up SBP and DBP were uniformly J-shaped, accentuated for the PCI patients and DBP, but excepting the CABG patients and DBP, for which the relation was linear and positive.
The results also indicate that adverse baseline conditions accounted for much of the increased risk of the CABG+PCI and CABG-only patients (Table 2). In contrast, the results suggest that the adverse baseline conditions accounted for essentially all the increased risk of the PCI patients. One likely explanation for this deduction is that the CABG+PCI and CABG-only patients had more advanced vascular disease in general, as was suggested by their increased incidence of peripheral vascular disease and stroke/transient ischemic attack at baseline and their greater pulse pressures during the study (Figure 1). Although our analysis indicates that differences in follow-up SBP and DBP individually were not responsible for an increased risk of adverse outcomes among the CABG+PCI and CABG-only patients, previous studies have shown that an increased pulse pressure does correlate with adverse outcomes.6 Another possible explanation is that the CABG procedure itself might have contributed to the subsequent adverse outcomes, although the INVEST was not designed to determine whether the increased risk was attributable to the procedure itself or to some other factor that was not available for analysis.
The relation of the adjusted risk of adverse outcome with follow-up SBP and DBP varied according to the revascularization strategy; however, for almost all, it was in the form of a J-shaped curve. The J-shaped curve was accentuated for the PCI patients and DBP; however, for CABG patients and DBP, the relation was linear and positive. The accentuated increase in adverse outcomes at a lower DBP for the PCI patients might have involved the consequent decrease in coronary perfusion, which occurs primarily during diastole. This decrease in coronary perfusion, especially if restenosis had occurred at the site of PCI, could precipitate chronic myocardial ischemia.7 Additionally, this decrease in perfusion might promote the development of thrombus, especially within a stent.8 Stent thrombosis frequently precipitates acute myocardial ischemia, and the incidence of death is high (31% to 45%).9,10
For CABG patients, the relation between the adjusted HR and DBP was not J-shaped but, rather, linear and positive. This linear and positive relation is especially noteworthy, considering those patients with previous CABG had less angina than those with previous PCI or CABG+PCI (Table 1). This finding might therefore have been the result of more complete and/or sustained revascularization such that those patients with previous CABG could both tolerate and simultaneously receive the benefits of a lower DBP. Regardless of the shape of the curve, the relation of the adjusted risk of major adverse outcomes with follow-up SBP and DBP suggests that managing BP to a specific target according to the type of revascularization in future studies could result in improved outcomes.
The present substudy had 2 important limitations. First, although the patients who had undergone previous revascularization represented a prespecified population of interest, the specific strategy of revascularization was not randomized. Second, randomization was not stratified to account for the baseline characteristic of revascularization status in general, nor was stratification of the revascularization procedure used. Therefore, imbalances between the patient groups might have been present beyond what we could adjust for in our analyses.
Acknowledgment
We thank Nancy Lanni, ELS, for editorial assistance, and Lisa A. Hamilton, MA, for the production of the figures.
The original INVEST study was supported by University of Florida (Gainesville, Florida) and Abbott Laboratories (Abbott Park, Illinois).
Dr. Messerli is an ad hoc consultant to, and speaker for, Abbott Laboratories (Abbott Park, Illinois). Drs. Cooper-DeHoff and Handberg have received a grant from Abbott Laboratories (Abbott Park, Illinois). Ms. Champion is an employee of, and has received stock/stock options from, Abbott Laboratories (Abbott Park, Illinois). Dr. Zhou is an employee of Abbott Laboratories (Abbott Park, Illinois). Dr. Pepine has received a grant from, and is an ad hoc consultant to, Abbott Laboratories (Abbott Park, Illinois).
References
- 1.Denardo SJ, Messerli FH, Gaxiola E, Aranda JM, Jr, Cooper-Dehoff RM, Handberg EM, Gong Y, Champion A, Zhou Q, Pepine CJ. Characteristics and outcomes of revascularized patients with hypertension: an INternational VErapamil SR-Trandolapril substudy. Hypertension. 2009;53:624–630. doi: 10.1161/HYPERTENSIONAHA.108.111542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pepine CJ, Handberg-Thurmond E, Marks RG, Conlon M, Cooper-DeHoff R, Volkers P, Zellig P. Rationale and design of the INternational VErapamil SR/Trandolapril Study (INVEST): an Internet-based randomized trial in coronary artery disease patients with hypertension. J Am Coll Cardiol. 1998;32:1228–1237. doi: 10.1016/s0735-1097(98)00423-9. [DOI] [PubMed] [Google Scholar]
- 3.Pepine CJ, Handberg EM, Cooper-DeHoff RM, Marks RG, Kowey P, Messerli FH, Mancia G, Cangiano JL, Garcia-Barreto D, Keltai M, Erdine S, Bristol HA, Kolb HR, Bakris GL, Cohen JD, Parmley WW. INVEST Investigators. A calcium antagonist vs a non-calcium antagonist hypertension treatment strategy for patients with coronary artery disease. The INternational VErapamil-Trandolapril Study (INVEST): a randomized controlled trial. JAMA. 2003;290:2805–2816. doi: 10.1001/jama.290.21.2805. [DOI] [PubMed] [Google Scholar]
- 4.D’Agostino RB., Jr Propensity scores in cardiovascular research. Circulation. 2007;115:2340–2343. doi: 10.1161/CIRCULATIONAHA.105.594952. [DOI] [PubMed] [Google Scholar]
- 5.Rubin DB. Using multivariate matched sampling and regression adjustment to control bias in observational studies. J Am Stat Assoc. 1979;74:318–328. [Google Scholar]
- 6.Domanski M, Mitchell G, Pfeffer M, Neaton JD, Norman J, Svendsen K, Grimm R, Cohen J, Stamler J MRFIT Research Group. Pulse pressure and cardiovascular disease-related mortality: follow-up study of the Multiple Risk Factor Intervention Trial (MRFIT) JAMA. 2002;287:2677–2683. doi: 10.1001/jama.287.20.2677. [DOI] [PubMed] [Google Scholar]
- 7.Hickey RF, Verrier ED, Baer RW, Vlahakes GJ, Fein G, Hoffman JI. A canine model of acute coronary artery stenosis: effects of deliberate hypotension. Anesthesiology. 1983;59:226–236. [PubMed] [Google Scholar]
- 8.Sukavaneshvar S, Rosa GM, Solen KA. Enhancement of stent-induced thromboembolism by residual stenoses: contribution of hemodynamics. Ann Biomed Eng. 2000;28:182–193. doi: 10.1114/1.243. [DOI] [PubMed] [Google Scholar]
- 9.Kuchulakanti PK, Chu WW, Torguson R, Ohlmann P, Rha SW, Clavijo LC, Kim SW, Bui A, Gevorkian N, Xue Z, Smith K, Fournadjieva J, Suddath WO, Satler LF, Pichard AD, Kent KM, Waksman R. Correlates and long-term outcomes of angiographically proven stent thrombosis with sirolimus- and paclitaxel-eluting stents. Circulation. 2006;113:1108–1113. doi: 10.1161/CIRCULATIONAHA.105.600155. [DOI] [PubMed] [Google Scholar]
- 10.Iakovou I, Schmidt T, Bonizzoni E, Ge L, Sangiorgi GM, Stankovic G, Airoldi F, Chieffo A, Montorfano M, Carlino M, Michev I, Corvaja N, Briguori C, Gerckens U, Grube E, Colombo A. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA. 2005;293:2126–2130. doi: 10.1001/jama.293.17.2126. [DOI] [PubMed] [Google Scholar]