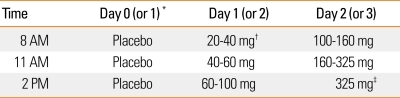
Table 2.
Oral aspirin challenges in patients with suspected aspirin exacerbated respiratory disease
*A placebo challenge can be conducted the week before. Alternatively, if the patient's baseline FEV1 is the same as their prior best value and they have not used their albuterol rescue inhaler in the past week, you can skip the one day placebo challenge. †Using a pill cutter, 81 mg ASA tablet can be cut into a half or a fourth. ‡If patient has not reacted to 325 mg of ASA, they will not react to 650 mg. Therefore, if no reaction occurs in 3 hours after ASA 325 mg call it a negative challenge.
(1) Measure FEV1 every hour and wait three hours between doses.
(2) FEV1 should be at least 1.5 L and > 60% of predicted.
(3) Reactions can be:
- Naso-ocular alone
- Naso-ocular and a 15% or > decline in FEV1 (Classic reaction)
- Lower respiratory reaction only (FEV1 declines by >20%)
- Laryngospasm with or without a, b, c (flat or notched inspiratory curve)
- Systemic reaction: hives, flush, gastric pain, hypotension
(4) Aspirin desensitization
- After a reaction has been treated and resolved go to b.
- Repeat the ASA provoking dose.
- If no reaction, continue to escalate the doses as above.
- At 325 mg of ASA, desensitization is always completed.
- Give 650 mg as first dose and then treat with 650 mg bid.