Abstract
Purpose
Bronchiectasis in children is still one of the most common causes of childhood mortality in developing countries. The aim of this study was to investigate the epidemiological characteristics, clinical features, underlying etiologic factors, and distinct change in the management of patients with bronchiectasis at Asan Medical Center Children's Hospital of Seoul.
Methods
A retrospective study of children diagnosed with bronchiectasis was conducted between January 1999 and December 2008. All patients underwent a comprehensive examination to identify etiologic factors. Data analysis in terms of age at onset, initial presenting symptoms, underlying etiology, distinct change in treatment, distribution of pulmonary involvement on computed tomography (CT), and causative microbiological flora triggering secondary infections was performed.
Results
The median age at the time of the diagnosis of bronchiectasis was 7.6 years (range, 2 months to 18 years). Persistent coughing was the most common symptom. The underlying etiologies identified in 79 patients (85.8%) included bronchiolitis obliterans (32.6%), childhood respiratory infection (20.6%), interstitial lung disease (17.3%), immunodeficiency (8.6%), and primary ciliary dyskinesia (4.3%). In 53 children (67%), the identified cause led to a distinct and individualized change in management. The distribution of CT abnormalities had no correlation with the underlying cause of bronchiectasis.
Conclusions
Selected Korean children with bronchiectasis were reviewed to identify diverse underlying etiologies. All children with bronchiectasis should be comprehensively investigated because identifying underlying causes may have a major impact on their management and prognosis.
Keywords: Bronchiectasis, etiology, bronchiolitis obliterans, infection, child
INTRODUCTION
Bronchiectasis is defined as permanent and abnormal dilation of the bronchi caused by destruction of the elastic and muscular components of the bronchial wall.1 In Western countries, cystic fibrosis (CF) is the most common cause of bronchiectasis, and other causes include various respiratory infections such as pneumonia, pertussis, measles, and tuberculosis. In particular, recurrent pneumonia is the major preceding factor leading to bronchial damage.2 Preexisting pneumonia is the most common cause of non-CF bronchiectasis.3 However, with vaccination and the widespread use of antibiotics, the incidence of post-infectious bronchiectasis has decreased. In contrast, clinicians are aware of the clinical implications of intrinsic defects or non-infective extrinsic factors. These include congenital defects, irritant aspiration, immunodeficiency, and mucociliary clearance dysfunction.4
High-resolution computed tomography (HRCT) is the most reliable noninvasive method for assessing the degree of bronchial wall dilatation, and thus bronchiectasis can be accurately diagnosed applying this technique.5,6 Detailed investigations are often conducted to determine the underlying cause of bronchiectasis, but little point exists unless they result in a change in management.
In this study, we sought to ascertain the clinical features of bronchiectasis, a distinct change in treatment according to the different causes of bronchiectasis, and the distribution of bronchiectasis on computed tomography (CT) and causative microbiological flora triggering secondary infection.
MATERIALS AND METHODS
Study subjects
We enrolled 92 Korean children aged 18 years or less who were confirmed with bronchiectasis by chest CT (including HRCT) from January 1999 to December 2008. They were retrospectively identified with bronchiectasis by the data warehouse system of Asan Medical Center Children's Hospital based on the International Classification of Disease-10.
Methods
The children's medical records were retrospectively reviewed using a standard form. At the clinical assessment, the following data were included: age at symptom onset, gender, initial symptoms, underlying etiology, and causative microbiological flora triggering a secondary infection. The distribution of bronchiectasis was identified in each lobe by CT scan, and widespread distribution was defined as the involvement of four or more lobes.4 The following tests were performed to determine the underlying etiology of the bronchiectasis: immunological tests including serum immunoglobulins (Ig), complement levels, lymphocyte subsets, and nitroblue tetrazolium test; serum α1-antitrypsin level; respiratory virus identification using nasopharyngeal aspirates; bacterial culture of sputum; Mantoux test; 24-hours esophageal pH monitoring; barium esophagography; bronchoscopic biopsy and bronchoalveolar lavage; electron microscopy of the nasal or bronchial mucosa cilia; sweat chloride test; and genetic studies. Each child was investigated to identify the underlying etiologic factor at the pediatric respirologist's discretion, and not all children underwent all these tests.
Lung function tests were performed on 68 patients (median age, 8.8 years; range, 5.1-16.3 years) according to the American Thoracic Society. Three acceptable maneuvers were performed, and the best value of forced expiratory volume in the first second (FEV1), its corresponding forced vital capacity (FVC), and FEV1/FVC ratio were recorded. Bronchiectasis was defined as idiopathic if extensive investigations failed to identify an underlying cause. This study was approved by the institutional review board of Asan Medical Center.
Statistical analysis
The demographical data and pulmonary function parameters were expressed as the median, range, and median percentage predicted, respectively.
RESULTS
Underlying etiology of bronchiectasis
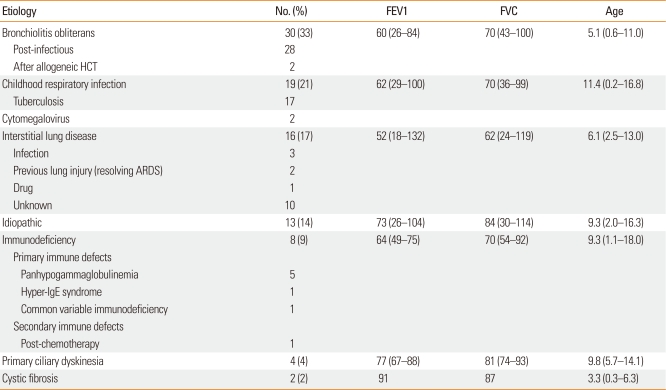
Ninety-two patients (47 males and 45 females; male to female ratio, 1.04:1) participated. The median age of the patients was 7.6 years (range, 2 months to 18 years). An etiology of bronchiectasis was identified in 79 patients (86%): bronchiolitis obliterans (33%, n=30), primary causative respiratory infection (21%, n=19), interstitial lung disease (17%, n=16), immunodeficiency (9%, n=8), primary ciliary dyskinesia (4%, n=4), and CF (2%, n=2). Of the 19 patients with respiratory infection, 17 (89.4%) had tuberculosis. Etiologic factors were not identified in 13 patients (14%). Among the 92 patients, 68 (74%) underwent a pulmonary function test: median FEV1, 63%; median FVC, 71% (Table 1).
Table 1.
Underlying etiology of bronchiectasis
FEV1 and FVC are presented as the median percentage predicted (range); age is presented as years (range).
FEV1, forced expiratory volume in the first second; FVC, forced vital capacity; HCT, hematopoietic cell transplantation; ARDS, acute respiratory distress syndrome; Ig, immunoglobulin.
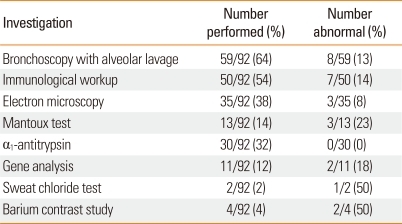
Tests for the diagnosis of bronchiectasis and their diagnostic yield are described in Table 2.
Table 2.
Investigation results
Data are presented as n/N (%).
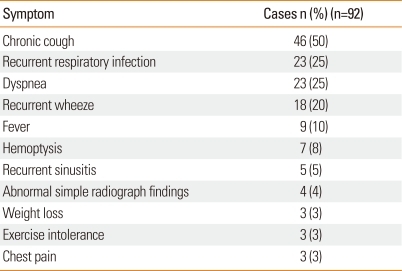
Main symptoms of bronchiectasis
At the time of the bronchiectasis diagnosis, the main symptoms were chronic cough (50%), repeated respiratory infection (25%), dyspnea (25%), recurrent wheeze (20%), fever (10%), and hemoptysis (8%) (Table 3).
Table 3.
Symptom at the time of bronchiectasis diagnosis
Specific treatments according to the causes of bronchiectasis
Of the 79 patients with identified etiologic factors, 53 were treated as follows according to the causes of bronchiectasis. Nineteen patients with bronchiolitis obliterans and eight patients with interstitial lung disease were treated with regular, high-dose methylprednisolone pulse therapy, six patients with immunodeficiency were treated with immunoglobulin replacement therapy, two patients with interstitial lung disease were treated with gancyclovir for cytomegalovirus infection, and one patient with interstitial lung disease due to paragonimiasis was treated with praziquantel.
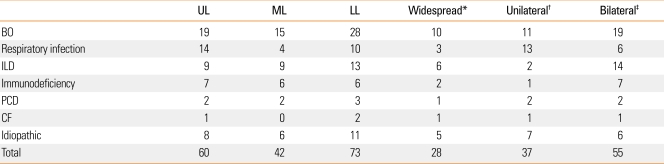
Distribution of bronchiectasis
The distribution of bronchiectasis assessed by chest CT is shown in Table 4. In patients with bronchiolitis obliterans, 10 of 30 (33%) showed widespread distribution, and the most common involved sites were the lower lobes (93%). In patients with respiratory infection, three of 19 (15%) showed widespread distribution, and this incidence was relatively lower than patients with other identified etiologic factors. Most patients with immunodeficiency showed an even distribution, but the statistical significance was difficult to evaluate due to the small number of cases. Twenty-eight patients (30%) showed a widespread distribution, suggesting that a considerable number of patients with bronchiectasis may have diffuse involvement. No significant association was observed between the distribution of bronchiectasis on chest CT and the causes of bronchiectasis.
Table 4.
Lobar involvement of bronchiectasis
Data are presented as n.
*Widespread: involvement of ≥4 lobes, †Unilateral: involvement only on side, ‡Bilateral: involvement on both sides.
UL, upper lobe involvement; ML, middle lobe involvement; LL, lower lobe involvement; BO, bronchiolitis obliterans; ILD, interstitial lung disease; PCD, primary ciliary dyskinesia; CF, cystic fibrosis.
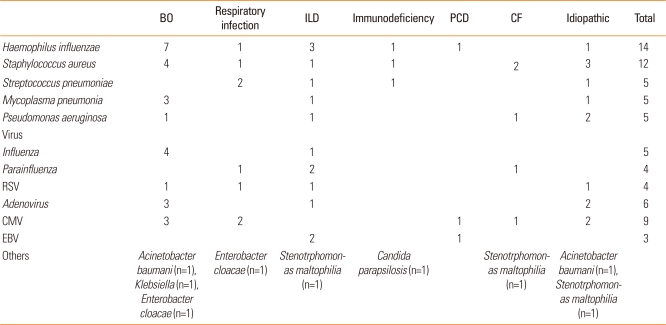
Causative secondary infection microorganisms in the patients with bronchiectasis
The microorganisms associated with each diagnosis are shown in Table 5. The most common organisms were Haemophilus influenza (15%), Staphylococcus aureus (13%), Streptococcus pneumonia (5%), Pseudomonas aeruginosa (5%), and Mycoplasma pneumonia (5%). Candida parapsilosis was isolated from one patient with immunodeficiency (Table 5).
Table 5.
Causative secondary infection microbiological flora
Data are presented as n.
BO, bronchiolitis obliterans; ILD, interstitial lung disease; PCD, primary ciliary dyskinesia; CF, cystic fibrosis; RSV, respiratory syncytial virus; CMV, cytomegalovirus; EBV, Epstein-Barr virus.
DISCUSSION
Bronchiectasis is a relatively frequent complication of lower respiratory tract infections and remains a persistent problem in children from developing countries.7,8 In developed countries, the incidence of bronchiectasis has decreased due to improvements in socioeconomic conditions and effective treatment of bacterial pneumonia (including the development of broadspectrum antibiotics).
The majority of the clinical data have indicated that bronchiectasis usually occurs in preschool-age children.9 In our study group, the mean onset age was 7 years and 7 months, and bronchiectasis occurred before 5 years of age in 39 patients (42%). As bronchiectasis presents no pathognomonic symptoms, identifying the etiologic factors based only on clinical symptoms was difficult in most cases. Other reports in the literature show that cough and sputum production were the most common symptoms,9,10 and in this study, chronic cough was the most common (Table 3). Considering that bronchiectasis occurred in 39 children (42%) aged ≤5 years, clinicians should be aware of the possibility that patients with chronic cough may have bronchiectasis regardless of their age. Wheezing is reported to be the main symptom in 20% of children with bronchiectasis.11 Eighteen patients (20%) in our study group had recurrent wheezing as a main symptom. Hemoptysis is a frequent symptom in adult patients with bronchiectasis, whereas it is a relatively uncommon in pediatric patients.7 In our study group, hemoptysis was noted in only seven patients (8%). Clubbing of the fingers was present in four patients (4%). Previous studies have reported that clubbing of the fingers occurs in 3-51% of patients.7,9,10
Bronchiectatic lesions are most commonly found in the lower lobes, probably because mucociliary clearance is facilitated by gravity in the upper lobes.7,11 In this study, bronchiectatic lesions were identified most commonly in the left lower lobe, followed by the right lower lobe and left upper lobe (Table 3). Idiopathic bronchiectasis occurs predominantly in the lower lobe, bronchiectasis due to primary ciliary dyskinesia in the middle lobe, and bronchiectasis due to hypogammaglobulinemia in the lower/middle lobe and lingual segment.12-14 However, whether the distribution of bronchiectasis might be sufficiently characteristic for a specific cause to be diagnosed is still controversial.15,16 In this study, as considerable overlap occurred in the distribution of bronchiectatic lesions on CT between different diseases, the cause of bronchiectasis could not be predicted based on the distribution of bronchiectatic lesions.
Comprehensive investigations are necessary to identify the cause of bronchiectasis in all children with bronchiectasis. Predisposing factors such as immunodeficiency, CF, and chronic aspiration should be considered according to clinical features. The importance of lung damage after respiratory infections, such as pneumonia, pertussis, measles, and tuberculosis, as a cause of bronchiectasis is difficult to estimate, but is still the most common cause in developing countries.2,8 In this study, although 40 patients (43%) had post-infectious bronchiectasis, no predisposing factors were found in 21 patients with the exclusion of tuberculosis (n=17) and cytomegalovirus infection (n=2), and a considerable number of patients with bronchiolitis obliterans and interstitial lung disease showed such findings. The etiological factor most commonly associated with bronchiectasis is childhood infection.17 With the widespread use of antibiotics and early regular vaccination, the incidence of infectious diseases has decreased. However, intrinsic abnormalities have been become more important predisposing factors. In this study, bronchiolitis obliterans was the most important underlying cause of bronchiectasis, suggesting that bronchiolitis obliterans due to viral or mycoplasma infections of the lower respiratory tract in children may induce progressive damage to the airway. However, as our hospital is a tertiary medical center, in which regional hospitals refer such patients, prospective studies are needed to confirm our result that bronchiolitis obliterans is the most common underlying etiology of childhood bronchiectasis. Furthermore, interstitial lung disease accounted for 17% of all patients, which requires more attention in clinical settings. Based on the result that bronchiectasis associated with bronchiolitis obliterans or interstitial lung disease occupied 50% of all patients, patients with bronchiolitis obliterans or interstitial lung disease should be closely observed on long-term follow-up.
The most common form of immunodeficiency associated with bronchiectasis in this study was panhypogammaglobulinemia (Table 1). Additionally, serum IgG subclass values can be low in patients with bronchiectasis, even in some cases with a normal total IgG level.18,19 Based on these results, IgG subclass levels should be measured even if total IgG is within the normal range.
In this study, three patients had ultrastructural abnormalities in the cilia on an electron microscopic examination and one had situs inversus. We assessed the ciliary ultrastructure of the nasal or bronchial mucosal membrane to diagnose primary ciliary dyskinesia. In patients with a normal ultrastructure of the cilia, primary ciliary dyskinesia can be diagnosed using low ciliary beat frequency.20 Therefore, to reduce the risk of misdiagnosis of primary ciliary dyskinesia, an examination of the ultrastructure of the cilia along with an analysis of ciliary beat frequency are mandatory. In this study, as we did not analyze ciliary beat frequency, some cases of primary ciliary dyskinesia might have been underestimated in the cases of unidentified underlying etiology.
Prolonged presence of a foreign body within the airway can result in the development of bronchiectasis.21,22 In this study, only four patients underwent a diagnostic workup for the presence of foreign bodies. Two patients were diagnosed with gastroesophageal reflux, which were excluded from the etiologic factors in this study due to the lack of its causal relationship with bronchiectasis.
Idiopathic bronchiectasis was found in 13 patients (14%). The incidence of idiopathic bronchiectasis in this study was lower than that of previous studies. Forty-eight percent of the subjects from a New Zealand cohort, despite extensive investigations, had no known underlying cause for their bronchiectasis.23 These results suggest that further diagnostic investigations are necessary to identify the etiologic factors and to treat bronchiectasis properly.
Our patients were treated by chest physical therapy and postural drainage with bronchodilator therapy, which may assist in mobilizing endobronchial secretions. Furthermore, short-term antibiotic therapy was performed to reduce the volume and bacterial density of sputum. Some studies have reported that such treatment modalities improve bronchial hyperreactivity and pulmonary function.24,25 Prompt and effective antibiotic therapy is essential to treat acute exacerbation of infection, and this therapy should be prescribed on the basis of bacterial culture and sensitivity tests.7,9 Although inflammation plays a crucial role in the pathogenesis of bronchiectasis, the use of anti-inflammatory agents has not been established. Inhaled high-dose fluticasone is effective for reducing the sputum inflammatory index in patients with bronchiectasis.26 In a Cochrane database, although inhaled steroids had no significant effect on treatment outcome, they significantly improved FEV1, FVC, and peak expiratory flow rate.27
To prevent bronchiectasis progression, accurately identifying and treating the underlying disease properly is important. However, how different treatment modalities affect the natural course of childhood bronchiectasis has not been studied.28 The necessity for surgical treatment has decreased due to early detection and improved drug therapy.2 Most studies have agreed that aggressive drug therapy should be conducted before surgical treatment, and some reports of effective surgical treatment have been published.29,30 However, no significant difference in clinical improvement between medical and surgical treatment was observed.19 In our study group, no patient was treated with surgery.
This study had some limitations. First, this was a cross-sectional study, which could not track the progression of the radiological abnormalities and disease course of bronchiectasis. Second, a possibility of referral bias existed. Some patients did not undergo all interventions. For example, some patients with definitive evidence of panhypogammaglobulinemia did not undergo further diagnostic tests. The prevalence of lung damage following infections seemed to be difficult to accurately estimate due to incorrect recall and reporting. Third, the time period between symptom onset and diagnosis was not quantified. Chest CTs may have been performed very late in the disease process when a spilling-over effect of the underlying disease may have occurred. For this reason, a correlation between the distribution of bronchiectatic lesions on chest CT scans and the etiologic factors was not determined.
In summary, the underlying causes of bronchiectasis in children were different from those in adults. CF is a common cause of bronchiectasis in childhood in Western countries. Compared to Western countries, this study showed that cystic fibrosis was uncommon and that bronchiolitis obliterans was the most important etiology. Thus, long-term follow-up is needed to detect the development of bronchiectasis in children with bronchiolitis obliterans. During childhood, intrinsic factors such as immunodeficiency are more implicated in the causes of bronchiectasis, so extensive investigations are needed to ascertain early implementation of therapy. Ultimately, this study suggests that identification of underlying causes through comprehensive investigation in children with bronchiectasis has a significant impact on their management and prognosis.
ACKNOWLEDGMENTS
This study was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry for Health & Welfare, Republic of Korea (A084144).
Footnotes
There are no financial or other issues that might lead to conflict of interest.
References
- 1.Balkanli K, Genç O, Dakak M, Gürkök S, Gözübüyük A, Caylak H, Yücel O. Surgical management of bronchiectasis: analysis and short-term results in 238 patients. Eur J Cardiothorac Surg. 2003;24:699–702. doi: 10.1016/s1010-7940(03)00497-4. [DOI] [PubMed] [Google Scholar]
- 2.Singleton R, Morris A, Redding G, Poll J, Holck P, Martinez P, Kruse D, Bulkow LR, Petersen KM, Lewis C. Bronchiectasis in Alaska native children: causes and clinical courses. Pediatr Pulmonol. 2000;29:182–187. doi: 10.1002/(sici)1099-0496(200003)29:3<182::aid-ppul5>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 3.Eastham KM, Fall AJ, Mitchell L, Spencer DA. The need to redefine non-cystic fibrosis bronchiectasis in childhood. Thorax. 2004;59:324–327. doi: 10.1136/thx.2003.011577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li AM, Sonnappa S, Lex C, Wong E, Zacharasiewicz A, Bush A, Jaffe A. Non-CF bronchiectasis: does knowing the aetiology lead to changes in management? Eur Respir J. 2005;26:8–14. doi: 10.1183/09031936.05.00127704. [DOI] [PubMed] [Google Scholar]
- 5.Hansell DM. Bronchiectasis. Radiol Clin North Am. 1998;36:107–128. doi: 10.1016/s0033-8389(05)70009-9. [DOI] [PubMed] [Google Scholar]
- 6.Brody AS. Cystic fibrosis: when should high-resolution computed tomography of the chest be obtained? Pediatrics. 1998;101:1071. doi: 10.1542/peds.101.6.1071. [DOI] [PubMed] [Google Scholar]
- 7.Ferkol TW, Davis PB. Bronchiectasis and bronchiolitis obliterans. In: Taussig LM, Landau LI, Le Souëf PN, Morgan WJ, Martinez FD, Sly PD, editors. Pediatric respiratory medicine. St Louis: Mosby; 1999. pp. 784–789. [Google Scholar]
- 8.Karakoc GB, Yilmaz M, Altintas DU, Kendirli SG. Bronchiectasis: still a problem. Pediatr Pulmonol. 2001;32:175–178. doi: 10.1002/ppul.1104. [DOI] [PubMed] [Google Scholar]
- 9.Brown MA, Lemen RJ. Bronchiectasis. In: Chernick V, Boat TF, Kendig EL, editors. Kendig's disorders of respiratory tract in children. 6th ed. Philadelphia: Saunders; 1998. pp. 538–552. [Google Scholar]
- 10.Lewinston NJ. Bronchiectasis. In: Hilman BC, editor. Pediatric respiratory disease: diagnosis and treatment. Philadelphia: Saunders; 1993. pp. 222–229. [Google Scholar]
- 11.Dagli E. Non cystic fibrosis bronchiectasis. Paediatr Respir Rev. 2000;1:64–70. doi: 10.1053/prrv.2000.0011. [DOI] [PubMed] [Google Scholar]
- 12.Reiff DB, Wells AU, Carr DH, Cole PJ, Hansell DM. CT findings in bronchiectasis: limited value in distinguishing between idiopathic and specific types. AJR Am J Roentgenol. 1995;165:261–267. doi: 10.2214/ajr.165.2.7618537. [DOI] [PubMed] [Google Scholar]
- 13.Kennedy MP, Noone PG, Leigh MW, Zariwala MA, Minnix SL, Knowles MR, Molina PL. High-resolution CT of patients with primary ciliary dyskinesia. AJR Am J Roentgenol. 2007;188:1232–1238. doi: 10.2214/AJR.06.0965. [DOI] [PubMed] [Google Scholar]
- 14.Curtin JJ, Webster AD, Farrant J, Katz D. Bronchiectasis in hypogammaglobulinaemia--a computed tomography assessment. Clin Radiol. 1991;44:82–84. doi: 10.1016/s0009-9260(05)80501-x. [DOI] [PubMed] [Google Scholar]
- 15.Lee PH, Carr DH, Rubens MB, Cole P, Hansell DM. Accuracy of CT in predicting the cause of bronchiectasis. Clin Radiol. 1995;50:839–841. doi: 10.1016/s0009-9260(05)83104-6. [DOI] [PubMed] [Google Scholar]
- 16.Cartier Y, Kavanagh PV, Johkoh T, Mason AC, Müller NL. Bronchiectasis: accuracy of high-resolution CT in the differentiation of specific diseases. AJR Am J Roentgenol. 1999;173:47–52. doi: 10.2214/ajr.173.1.10397098. [DOI] [PubMed] [Google Scholar]
- 17.King P, Holdsworth S, Freezer N, Holmes P. Bronchiectasis. Intern Med J. 2006;36:729–737. doi: 10.1111/j.1445-5994.2006.01219.x. [DOI] [PubMed] [Google Scholar]
- 18.Hill SL, Mitchell JL, Burnett D, Stockley RA. IgG subclasses in the serum and sputum from patients with bronchiectasis. Thorax. 1998;53:463–468. doi: 10.1136/thx.53.6.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karadag B, Karakoc F, Ersu R, Kut A, Bakac S, Dagli E. Non-cystic-fibrosis bronchiectasis in children: a persisting problem in developing countries. Respiration. 2005;72:233–238. doi: 10.1159/000085362. [DOI] [PubMed] [Google Scholar]
- 20.Buchdahl RM, Reiser J, Ingram D, Rutman A, Cole PJ, Warner JO. Ciliary abnormalities in respiratory disease. Arch Dis Child. 1988;63:238–243. doi: 10.1136/adc.63.3.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karakoc F, Cakir E, Ersu R, Uyan ZS, Colak B, Karadag B, Kiyan G, Dagli T, Dagli E. Late diagnosis of foreign body aspiration in children with chronic respiratory symptoms. Int J Pediatr Otorhinolaryngol. 2007;71:241–246. doi: 10.1016/j.ijporl.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 22.Karakoç F, Karadağ B, Akbenlioğlu C, Ersu R, Yildizeli B, Yüksel M, Dali E. Foreign body aspiration: what is the outcome? Pediatr Pulmonol. 2002;34:30–36. doi: 10.1002/ppul.10094. [DOI] [PubMed] [Google Scholar]
- 23.Edwards EA, Metcalfe R, Milne DG, Thompson J, Byrnes CA. Retrospective review of children presenting with non cystic fibrosis bronchiectasis: HRCT features and clinical relationships. Pediatr Pulmonol. 2003;36:87–93. doi: 10.1002/ppul.10339. [DOI] [PubMed] [Google Scholar]
- 24.Tsang KW, Ho PI, Chan KN, Ip MS, Lam WK, Ho CS, Yuen KY, Ooi GC, Amitani R, Tanaka E. A pilot study of low-dose erythromycin in bronchiectasis. Eur Respir J. 1999;13:361–364. doi: 10.1183/09031936.99.13236199. [DOI] [PubMed] [Google Scholar]
- 25.Koh YY, Lee MH, Sun YH, Sung KW, Chae JH. Effect of roxithromycin on airway responsiveness in children with bronchiectasis: a double-blind, placebo-controlled study. Eur Respir J. 1997;10:994–999. doi: 10.1183/09031936.97.10050994. [DOI] [PubMed] [Google Scholar]
- 26.Tsang KW, Ho PL, Lam WK, Ip MS, Chan KN, Ho CS, Ooi CC, Yuen KY. Inhaled fluticasone reduces sputum inflammatory indices in severe bronchiectasis. Am J Respir Crit Care Med. 1998;158:723–727. doi: 10.1164/ajrccm.158.3.9710090. [DOI] [PubMed] [Google Scholar]
- 27.Kolbe J, Wells A, Ram FS. Inhaled steroids for bronchiectasis. Cochrane Database Syst Rev. 2000:CD000996. doi: 10.1002/14651858.CD000996. [DOI] [PubMed] [Google Scholar]
- 28.Callahan CW, Redding GJ. Bronchiectasis in children: orphan disease or persistent problem? Pediatr Pulmonol. 2002;33:492–496. doi: 10.1002/ppul.10104. [DOI] [PubMed] [Google Scholar]
- 29.Kutlay H, Cangir AK, Enön S, Sahin E, Akal M, Güngör A, Ozdemir N, Kavukçu S. Surgical treatment in bronchiectasis: analysis of 166 patients. Eur J Cardiothorac Surg. 2002;21:634–637. doi: 10.1016/s1010-7940(02)00053-2. [DOI] [PubMed] [Google Scholar]
- 30.Sirmali M, Karasu S, Türüt H, Gezer S, Kaya S, Taştepe I, Karaoğl anoğlu N. Surgical management of bronchiectasis in childhood. Eur J Cardiothorac Surg. 2007;31:120–123. doi: 10.1016/j.ejcts.2006.10.021. [DOI] [PubMed] [Google Scholar]