Abstract
The physiopathology of venous symptoms, such as pain, leg heaviness or swelling sensations, in chronic venous disease (CVD) remains unclear. Localized release of proinflammatory mediators appears to play a key role but the presence of nociceptors sensitive to inflammatory mediators, such as unmyelinated C fibres, needs to be demonstrated. The present study included 10 patients with documented CVD who underwent surgery for saphenectomy. For each patient, five segments of the great saphenous vein were immunostained with anti-S100 protein and anti-CD45 to identify nerve fibres and inflammatory cells, respectively. Light microscopy was completed by electron microscopy. In all patients, S100 immunopositive nerve fibres and CD45 immunopositive cells were observed. Under an electron microscope, advanced signs of wall remodelling were systematically observed. The density of nerve fibres was low and variable from one sample to another. Unmyelinated C fibres were mainly located in the external part of the media and to a lesser extent in the internal part of the adventitia. Inflammatory cells, mainly histiocytes, were scattered in the media. Mast cells were observed in three patients. In conclusion, unmyelinated C fibres and inflammatory cells are present in the varicose saphenous vein wall. Their linked roles in symptoms of CVD should be further explored.
Keywords: C fibres, Chronic venous disease, Electron microscopy, Immunohistochemistry, Inflammatory cells, Varicose veins
Chronic venous disease (CVD) is a common disorder with a significant impact on quality of life and health care costs in western countries (1,2). Occurrence of symptoms, which are variously described as pain, heaviness, tension, ache and cramp, and itching, tingling or swelling sensations, is high, occurring in close to 95% of CVD-affected patients (3). Venous symptoms are of great importance in medical practice, but their mechanisms remain yet to be considered. Patients with CVD report variable pain and express it as a visceral discomfort, which is in favour of involvement of unmyelinated C fibres (4,5). Moreover, body shape perception distortions, such as a swelling sensation that is frequently reported by patients with CVD, are known to involve C fibres (6,7). One concept in the literature is based on the presence of mast cells and macrophages inside the wall of enlarged varicose veins that are able to activate nociceptors and subsequently generate painful sensations. The aim of the present study was to observe C fibres, their localization and their relationship with inflammatory cells in varicose vein walls.
METHODS
Patient population
The present study was approved by the local ethics committee and all patients provided signed, informed consent. Ten patients suffering from highly symptomatic CVD (pain intensity score of at least 30 mm on a 100 mm visual analogue scale [VAS]) and undergoing resection of the great saphenous vein by saphenectomy were included. According to the clinical, etiological, anatomical and pathophysiological (CEAP) classification, five patients belonged to the C2 to C3 group (varicose vein with or without edema) and five patients belonged to the C4 to C5 group (patients presenting with CVD-related skin changes and/or a healed venous ulcer). Reflux of the great saphenous vein was present in all patients. Mean age ranged from 33 to 65 years (mean [± SD] 51.7±10.9 years), and 80% of the patients were women. All were nonsmokers or had stopped smoking, and none reported regular alcohol consumption. Varicose vein disease had been diagnosed one to 10 months previously (mean 3.9 months), and 80% of patients had a family history of CVD. None of the patients had previously undergone surgery for CVD. All patients were experiencing symptoms of CVD (100% reported pain and 90% reported leg heaviness) and, in most cases, had experienced them for one to five years. Pain intensity, assessed on a 100 mm VAS at inclusion, ranged from 29 mm to 67 mm and tended to be higher in C4 to C5 patients (mean 48.8±17.0 mm) than in C2 to C3 patients (mean 42.0±6.7 mm).
Procedure
Each vein was processed within 15 min after resection. The vein wall was first sectioned into five segments. From each segment, a thin biopsy specimen involving the whole vessel wall thickness was immediately immersed in a fresh buffered solution of 2.5% glutaraldehyde for electron microscopy. The remaining tissue from each of the five segments was fixed in 10% neutral buffered formalin solution for light microscopy.
Specimens for electron microscopy were postfixed in osmium tetroxide, and epon-embedded semithin sections were prepared for preliminary light microscope examination. Ultrathin sections were prepared for ultrastructural examination under a Tecnai electron microscope (FEI Company, USA).
Specimens intended for light microscopy were embedded in paraffin, and 2.5 μm thick sections were stained with hematoxylin-eosin-saffron. On deparaffinized sections using the standard peroxidase-antiperoxidase technique, immunostaining was performed with protein S100 antibody (polyclonal rabbit anti-S100; DakoCytomation, Denmark A/S) for the labelling of Schwann cells (ie, nerve fibres) and CD45 antibody (monoclonal mouse antihuman CD45, clones 2B11 + PD7/26, DakoCytomation) for the labelling of panleukocyte cells. Results were expressed using a semiquantitative quotation at original magnification ×20, over 1 mm2: 0 indicated no labelled element, + indicated fewer than two labelled elements, ++ indicated two to five labelled elements and +++ indicated more than five labelled elements.
RESULTS
Histology and immunohistochemistry
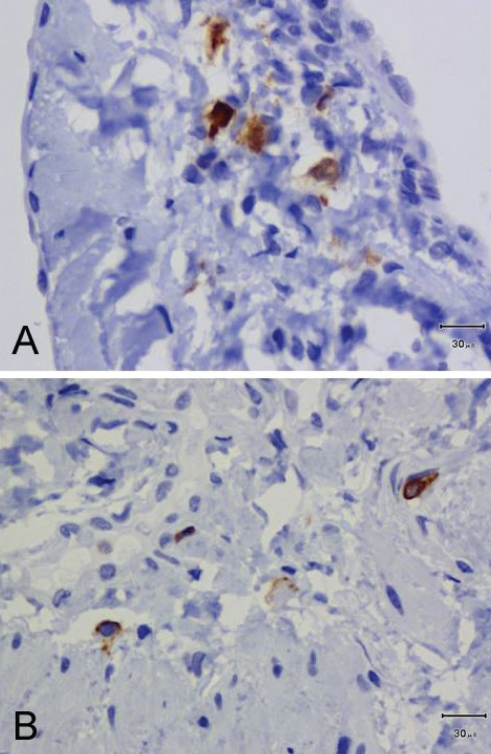
S100-immunopositive nerve fibres were observed in all patients (Table 1 and Figure 1A). Their density was low: 59.1% of observed specimens exhibited no or fewer than two nerve fibres, and two to five nerve fibres were found in 40.8% of 49 available samples (Table 2). In most sections, nerve fibres were scattered in the media. Some nerve fibres were identified in the vicinity of the vasa vasorum, close to the adventitia. CD45-immunopositive cells were observed in all patients at low density (Figure 1B). None or fewer than two cells per field were observed in 57.1% of 49 available samples (Table 2). The number of specimens with a low density of nerve fibres (fewer than two nerve fibres per sample) tended to be higher in the C4 to C5 CEAP group than in the C2 to C3 CEAP group (Table 1), while no difference in the density of CD45-immunopositive elements was observed between the two CEAP groups.
TABLE 1.
Conventional histology and clinical, etiological, anatomical and pathophysiological classification of patients
| C2 to C3 (N=5) (n=5/subject) | C4 to C5 (N=5) (n=5/subject) | ||
|---|---|---|---|
| S100 immunostaining | |||
| Presence of positive elements | N (% of subjects) | 5 (100.0) | 5 (100.0) |
| n (% of samples) | 25 (100.0) | 18 (75.0)* | |
| Nerve fibres per sample | |||
| None | n (% of samples) | 0 (0) | 6 (25.0)* |
| <2 | n (% of samples) | 11 (44.0) | 12 (50.0)* |
| 2–5 | n (% of samples) | 14 (56.0) | 6 (25.0)* |
| CD45 immunostaining | |||
| Presence of positive elements | N (% of subjects) | 5 (100.0) | 5 (100.0) |
| n (% of samples) | 24 (96.0) | 21 (87.5)* | |
| Nerve fibres per sample | |||
| None | n (% of samples) | 1 (4.0) | 3 (12.5)* |
| <2 | n (% of samples) | 13 (52.0) | 11 (45.8)* |
| 2–5 | n (% of samples) | 11 (44.0) | 10 (41.7)* |
One sample could not be interpreted. N Number of subjects; n Number of specimens
Figure 1).
Immunohistochemistry in patient 2. S100 immunopositive nerve fibres are present in the media (A) and CD45 immunopositive leukocytes are few and scattered in the vein wall (B). Scale bar = 30 μm in A and B
TABLE 2.
Density of immunopositive elements (n=49 samples)
| Staining |
Density of elements, % |
||
|---|---|---|---|
| None | <2 per field | 2 to 5 per field | |
| S100 (nerve fibres) | 12.2 | 46.9 | 40.8 |
| CD45 (panleukocyte cells) | 8.2 | 48.9 | 42.9 |
Electron microscopy
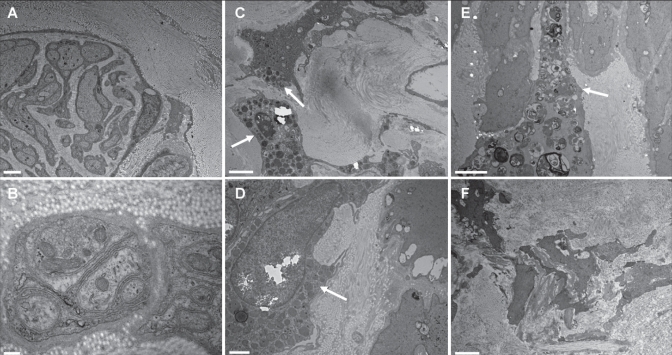
Unmyelinated C fibres were seen in eight of 10 patients (Figures 2A and 2B). These C fibres were rare and located mainly inside the media and the internal part of the adventitia (Table 3). A close relationship with smooth muscle cells was observed in one patient. In some samples, unmyelinated axons were organized in bundles or clusters (Figure 2A).
Figure 2).
Electron micrographs of varicose veins. Unmyelinated C fibres are identified between the media and adventitia (A and B) in patients 5 and 9, respectively. Mast cells (arrows) are present in the media of patient 2 (C and D); one of these mast cells is degranulated (D). A histiocytic macrophage overloaded with products of degradation (arrow) is in the vicinity of smooth muscle fibres in patient 6 (E). Smooth muscle fibres of the media from patient 2 appear disintegrated into pieces and scattered in an abundant collagen accumulation (F). Scale bar = 2 μm in A, C, E and F; scale bar = 200 nm in B; scale bar = 1 μm in D
TABLE 3.
Electron microscope study of unmyelinated C fibres
| Patients | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Number of samples* with C fibres | 2 | 2 | 3 | 3 | 1 | 2 | 2 | 2 | 0 | 0 |
| Inside the intima | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | – | – |
| Inside the adventitia | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | – | – |
| Inside the media | 1 | 2 | 3 | 2 | 0 | 2 | 1 | 1 | – | – |
| Close to SMCs | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | – | – |
| Close to elastic fibres | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | – | – |
Five samples observed for each patient. SMCs Smooth muscle cells
In three patients, mast cells were mainly located in the media, often close to the adventitia, but not in close contact with C fibres (Figures 2C and 2D). Interestingly, a mast cell appeared degranulated in one sample (Figure 2D). Histiocytes, with the shape of macrophages rather than monocytes, were present in all patients (98% of samples) (Figure 2E). Those cells correspond to the CD45-immunostained cells observed in light microscopy and have been identified as autonomic histiocytes (ie, likely to come from the blood stream), as opposed to dedifferentiated smooth muscle cells. Neutrophils and lymphocytes were not observed.
In all patients, typical aspects of wall remodelling (ie, accumulation of collagen bundles and lipids, and dedifferentiated smooth muscle cells) were extensively present (Figure 2F).
DISCUSSION
The main result of the present study was the identification of unmyelinated C fibres in the walls of varicose veins, seemingly arranged as a wide mesh arising from the adventitia and spreading out into the external part of the media. This setting suggests the existence of a neurological component able to diffuse the pain signal from the vein into the spinal cord and, finally, to the brain. Inflammatory cells and particularly mast cells that could be responsible for activation of those C fibres were also found in the pathological vein wall.
Little is known about the C fibres’ innervation and relationship with inflammatory cells in human blood vessels, particularly in veins (8–10). One of the first studies (11) conducted on nerves supplying the saphenous vein in cats reported that unmyelinated fibres accounted for two-thirds of the afferent fibres. C fibres are mechanical, chemical and thermal nociceptors and, incidentally, are also known as polymodal nociceptors. Primary afferent C fibres support prodromic functions to signal pain, heat, cold and itch through the dorsal horn of the spinal cord. Peripherally, efferent C fibres act locally to stimulate circulatory and immune responses, exerting antidromic functions through release of neuropeptides such as substance P and calcitonin gene-related peptide (CGRP). Beyond vessel innervation (4), C fibres are known to be implicated in the sensory innervation of the skin (12), visceral organs (13) and dental pulp (14).
In our study, C fibres were observed in all patients, mainly in the media and adventitia, in agreement with previous results reporting a strong S100 immunostaining of nerve fibres along the wall of the vasa vasorum and in the media (9). No positive staining was found within the intima. The density of C fibres was low. Consequently, no C fibres were revealed by electron microscopy in two patients, whereas S100 immunopositive nerve fibres were observed in all patients by light microscopy. Unfortunately, for ethical and practical reasons, it was not possible to compare the density of C fibres with that of normal saphenous vein specimens in our study. Nerve fibre density is related to vessel territories concerned with organ functions. In human mesenteric veins, the density of sympathetic nerve fibres is roughly sixfold higher than in arteries (15), and they appear as unmyelinated fibres. Their higher density may reflect the need for fine control of vascular tone for mesenteric capacitance control because of their thick muscular media. In contrast, in dental pulp vasculature, the density of vasomotor adrenergic innervation received by venules is four times lower than that received by arterioles (16). C fibre bundles or clusters, which are often located close to the vasa vasorum and adventitia (15,17,18), were also observed in some of our specimens.
We observed inflammatory cells in the varicose vein wall. These cells were mainly histiocytes, which can be considered to originate from the blood stream rather than having dedifferentiated from smooth muscle cells, according to CD45 immunostaining. They were observed in close contact with altered smooth muscle cells and were overloaded with products of degradation, which highlights their involvement in the process of remodelling, as previously reported (19). Macrophage/monocyte infiltration has been extensively studied in CVD, particularly its role in valvular vein infiltration by collagenase release, and it is considered to be a key factor in damage leading to valvular incompetence (19,20). In previous reports, as in the present study, no lymphocyte and neutrophil infiltration was noted.
Previous studies have compared varicose veins with control veins in which no mast cells were seen (19,21). The low rate of mast cell infiltration compared with macrophage infiltration observed in our study is also in agreement with a previous report (19) in which macrophage monocytes were roughly fivefold more common than mast cells. A mast cell was degranulated in one of our specimens. Even if no direct relationship between mast cells and C fibres was established in our study, it has been shown that neuropeptides, including CGRP, can induce mast cell content release in rat skin (22). In another model of electrical stimulation of sensory nerves in rat skin, antidromic extended stimulation of C fibres induced mast cell degranulation and histamine release. According to prolonged peptidergic fibre activation, it was suggested that mast cells are not involved in the initial stages of inflammation and vascular permeability responses in skin, but could reinforce it (23). On the other hand, mast cell degranulation corresponds to the release of vasoactive substances (histamine and tryptase) or cytokine tumour necrosis factor-alpha, which are able to induce endothelial L-selectin and consequently recruit leukocytes. This supports their involvement in earlier events of varicose vein formation (24). C fibres and mast cells may form a functional unit that could contribute significantly to mechanisms arising at earlier and/or later stages of CVD.
The type and intensity of vessel remodelling reported in all our patients are in agreement with previous studies (19,21) on varicose saphenous veins. Evidence of remodelling consisted of deposition of collagen in connective tissue, disruption of smooth muscle cell architecture and macrophage-monocyte infiltration. This histological pattern is different from that observed in aging people, in whom extensive fibrosis is present in the adventitia and intima rather than in the media, which is specifically altered in varicose veins (25).
The aim of the study was to confirm the presence of C fibres in varicose saphenous veins from highly symptomatic patients. One of the inclusion criteria of our study was a pain intensity score of at least 30 mm on a 100 mm VAS. Because all patients were highly symptomatic, it was not possible to assess whether there was any relationship between C fibre density and pain intensity. It would be interesting to assess whether C fibres are similarly present in nonvaricose veins and in varicose veins of asymptomatic patients. Because the intensity of pain in CVD is not correlated with the severity of reflux measured with Doppler scanning in lower limb veins (26), this suggests that the primary activation site of venous and/or perivenous nociceptors of C fibres may not be located in large venous vessels, but is probably located in the microcirculation. C fibre density was low, and it was not possible to demonstrate on electron microscopy close contact between C fibres and inflammatory cells. Nevertheless, this does not rule out the hypothesis of an active role of C fibres in the physiopathology of CVD through release of neuropeptides (substance P and CGRP) acting directly or in relation with inflammatory cell activation. The absence of appropriate controls, for ethical reasons, is one limitation of our study. Location, type and density of inflammatory cell infiltration correspond with previous reports, as does intensive wall remodelling. Coexistence of unmyelinated C fibres and mast cells was demonstrated.
Acknowledgments
This work was supported by a grant from Institut de Recherches Internationales Servier IRIS n° CL1-ANGIO-001/2008.
REFERENCES
- 1.Bergan JJ, Schmid-Schonbein GW, Smith PD, Nicolaides AN, Boisseau MR, Eklof B. Chronic venous disease. N Engl J Med. 2006;355:488–98. doi: 10.1056/NEJMra055289. [DOI] [PubMed] [Google Scholar]
- 2.Weingarten MS. State-of-the-art treatment of chronic venous disease. Clin Infect Dis. 2001;32:949–54. doi: 10.1086/319360. [DOI] [PubMed] [Google Scholar]
- 3.Chiesa R, Marone EM, Limoni C, Volonte M, Schaefer E, Petrini O. Effect of chronic venous insufficiency on activities of daily living and quality of life: Correlation of demographic factors with duplex ultrasonography findings. Angiology. 2007;58:440–9. doi: 10.1177/0003319706292012. [DOI] [PubMed] [Google Scholar]
- 4.Arndt JO, Klement W. Pain evoked by polymodal stimulation of hand veins in humans. J Physiol. 1991;440:467–78. doi: 10.1113/jphysiol.1991.sp018719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klement W, Arndt JO. Pain but no temperature sensations are evoked by thermal stimulation of cutaneous veins in man. Neurosci Lett. 1991;123:119–22. doi: 10.1016/0304-3940(91)90172-p. [DOI] [PubMed] [Google Scholar]
- 6.Danziger N. [Pathophysiology of pain in venous disease] J Mal Vasc. 2007;32:1–7. doi: 10.1016/j.jmv.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Paqueron X, Leguen M, Rosenthal D, Coriat P, Willer JC, Danziger N. The phenomenology of body image distortions induced by regional anaesthesia. Brain. 2003;126:702–12. doi: 10.1093/brain/awg063. [DOI] [PubMed] [Google Scholar]
- 8.Amenta F, Cavallotti C, Dotta F, Ferrante F, Spinelli F, Vatrella F. The autonomic innervation of the human greater saphenous vein. Acta Histochem. 1983;72:111–6. doi: 10.1016/S0065-1281(83)80017-8. [DOI] [PubMed] [Google Scholar]
- 9.Herbst WM, Eberle KP, Ozen Y, Hornstein OP. The innervation of the great saphenous vein: An immunohistochemical study with special regard to regulatory peptides. Vasa. 1992;21:253–7. [PubMed] [Google Scholar]
- 10.Loesch A, Dashwood MR. On the sympathetic innervation of the human greater saphenous vein: Relevance to clinical practice. Curr Vasc Pharmacol. 2009;7:58–67. doi: 10.2174/157016109787354150. [DOI] [PubMed] [Google Scholar]
- 11.Michaelis M, Goder R, Habler HJ, Janig W. Properties of afferent nerve fibres supplying the saphenous vein in the cat. J Physiol. 1994;474:233–43. doi: 10.1113/jphysiol.1994.sp020016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.White DM, Leah JD, Zimmermann M. The localization and release of substance P and calcitonin gene-related peptide at nerve fibre endings in rat cutaneous nerve neuroma. Brain Res. 1989;503:198–204. doi: 10.1016/0006-8993(89)91664-8. [DOI] [PubMed] [Google Scholar]
- 13.Yvonne-Tee GB, Rasool AH, Halim AS, Rahman AR. Noninvasive assessment of cutaneous vascular function in vivo using capillaroscopy, plethysmography and laser-Doppler instruments: Its strengths and weaknesses. Clin Hemorheol Microcirc. 2006;34:457–73. [PubMed] [Google Scholar]
- 14.Gazelius B, Edwall B, Olgart L, Lundberg JM, Hokfelt T, Fischer JA. Vasodilatory effects and coexistence of calcitonin gene-related peptide (CGRP) and substance P in sensory nerves of cat dental pulp. Acta Physiol Scand. 1987;130:33–40. doi: 10.1111/j.1748-1716.1987.tb08108.x. [DOI] [PubMed] [Google Scholar]
- 15.Birch DJ, Turmaine M, Boulos PB, Burnstock G. Sympathetic innervation of human mesenteric artery and vein. J Vasc Res. 2008;45:323–32. doi: 10.1159/000119095. [DOI] [PubMed] [Google Scholar]
- 16.Okamura K, Kobayashi I, Matsuo K, et al. An immunohistochemical and ultrastructural study of vasomotor nerves in the microvasculature of human dental pulp. Arch Oral Biol. 1995;40:47–53. doi: 10.1016/0003-9969(94)00147-4. [DOI] [PubMed] [Google Scholar]
- 17.Henderson J, Terenghi G, McGrouther DA, Ferguson MW. The reinnervation pattern of wounds and scars may explain their sensory symptoms. J Plast Reconstr Aesthet Surg. 2006;59:942–50. doi: 10.1016/j.bjps.2005.11.038. [DOI] [PubMed] [Google Scholar]
- 18.Paulsen F, Hallmann U, Paulsen J, Thale A. Innervation of the cavernous body of the human efferent tear ducts and function in tear outflow mechanism. J Anat. 2000;197:177–87. doi: 10.1046/j.1469-7580.2000.19720177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sayer GL, Smith PD. Immunocytochemical characterisation of the inflammatory cell infiltrate of varicose veins. Eur J Vasc Endovasc Surg. 2004;28:479–83. doi: 10.1016/j.ejvs.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 20.Ono T, Bergan JJ, Schmid-Schonbein GW, Takase S. Monocyte infiltration into venous valves. J Vasc Surg. 1998;27:158–66. doi: 10.1016/s0741-5214(98)70303-9. [DOI] [PubMed] [Google Scholar]
- 21.Yamada T, Tomita S, Mori M, Sasatomi E, Suenaga E, Itoh T. Increased mast cell infiltration in varicose veins of the lower limbs: A possible role in the development of varices. Surgery. 1996;119:494–7. doi: 10.1016/s0039-6060(96)80256-x. [DOI] [PubMed] [Google Scholar]
- 22.Theoharides TC, Singh LK, Boucher W, et al. Corticotropin-releasing hormone induces skin mast cell degranulation and increased vascular permeability, a possible explanation for its proinflammatory effects. Endocrinology. 1998;139:403–13. doi: 10.1210/endo.139.1.5660. [DOI] [PubMed] [Google Scholar]
- 23.Kowalski ML, Kaliner MA. Neurogenic inflammation, vascular permeability, and mast cells. J Immunol. 1988;140:3905–11. [PubMed] [Google Scholar]
- 24.Walsh LJ, Trinchieri G, Waldorf HA, Whitaker D, Murphy GF. Human dermal mast cells contain and release tumor necrosis factor alpha, which induces endothelial leukocyte adhesion molecule 1. Proc Natl Acad Sci U S A. 1991;88:4220–4. doi: 10.1073/pnas.88.10.4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leu HJ, Vogt M, Pfrunder H. Morphological alterations of non-varicose and varicose veins. (A morphological contribution to the discussion on pathogenesis of varicose veins) Basic Res Cardiol. 1979;74:435–44. doi: 10.1007/BF01908395. [DOI] [PubMed] [Google Scholar]
- 26.Howlader MH, Smith PD. Symptoms of chronic venous disease and association with systemic inflammatory markers. J Vasc Surg. 2003;38:950–4. doi: 10.1016/s0741-5214(03)00600-1. [DOI] [PubMed] [Google Scholar]