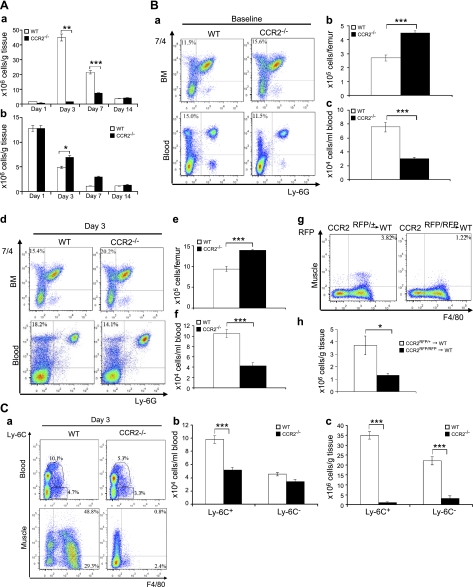
Figure 4.
CCR2 deficiency blocked MO/MP recruitment into injured muscle.. A) Flow cytometry showed a markedly increased number of intramuscular macrophages in wild-type mice at d 3 and 7, with the peak at d 3 (a). The number of intramuscular macrophages in Ccr2−/− mice was also increased after injury (a) but was significantly reduced at d 3 and 7 as compared with wild-type mice. The number of neutrophils in Ccr2−/− mice was increased at d 3 as compared with wild-type mice (b). B) Flow cytometry at baseline showed that the percentage of 7/4+Ly-6G− MOs/MPs (a) was increased in bone marrow but reduced in blood in Ccr2−/− mice as compared with wild-type controls. At d 3 postinjury, the percentage of 7/4+Ly-6G− MOs/MPs (d) was also increased in bone marrow and reduced in blood in Ccr2−/− mice. The numbers of 7/4+Ly-6G− MOs/MPs per femur were significantly higher in bone marrow (BM; b, e) but lower in blood (c, f) at baseline (b, c) and d 3 (e, f) in Ccr2−/− mice than those in wild-type mice. Bone marrow monocyte transfer showed that the number of Ccr2RFP/+ monocytes recruited into wild-type injured muscle at d 3 was significantly higher than that of Ccr2RFP/RFP monocytes (lacking CCR2) (g, h). C) Flow cytometry at d 3 showed that the numbers of F4/80+ MOs/MPs were reduced in blood and in injured muscle of Ccr2−/− mice (a), among which the Ly-6C+ but not the Ly-6C− subset was significantly reduced in blood (b). However, both Ly-6C+ and Ly-6C− subsets were significantly reduced in injured muscle of Ccr2−/− mice (c). n = 5–7 mice/group/experiment. *P < 0.05; **P < 0.01; ***P < 0.001.