Mutually reinforcing studies reported by Taniguchi et al.1 in this issue of Nature Medicine and elsewhere by Murase et al.2 have provided important insights into transplantation immunology. Both investigations show that pluripotent hematolymphopoietic stem cells reside in the liver of mature rodents, and by inference in other organs. The crucial evidence supporting this phenomenon is provided by the reconstitution of supralethally irradiated mice (9.5 Gy) with stem cells purified from adult mouse livers1 and the rescue of irradiated rats by the direct expedient of liver transplantation2. With either approach, all hematolymphopoietic lineages are restored in the recipients.
Two historical contributions preceding these results are noteworthy, both also involving supralethal irradiation of recipients before transplantation. In the first report, Hays et al.3 describe multilineage reconstitution in mice with cultured syngeneic hepatic non-parenchymal cells (NPCs) obtained from adult mouse liver. (Reconstitution was most marked when NPCs were cultured from regenerating liver.) More recently, Decker et al. emphasized the equivalence of reconstitution in recipients transplanted with cultured syngeneic liver NPCs versus bone marrow cells. Using sophisticated contemporary technology, Taniguchi et al. have greatly extended these observations, showing that the frequency of pluripotent stem cells in the mouse liver is at least half that in bone marrow and about five times that in peripheral blood. As few as 500 of these sorted cells isolated from the NPCs of adult mouse liver allow full reconstitution of irradiated recipients.
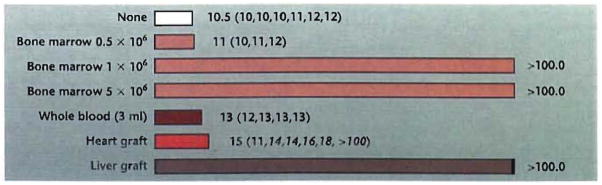
The organ transplant experiments of Murase et al. provide congruent evidence that the liver contains pluripotent hematopoietic stem cells. The multilineage reconstitution and permanent survival following orthotopic transplantation of thoroughly flushed livers to irradiated rat recipients is equivalent to that obtained after transplant of 106 unaltered syngeneic bone marrow cells (Fig. 1). Heterotopic heart transplantation also has a significant effect on postirradiation survival. Prolongation of survival is less dramatic than that seen after liver transplant but is similar to that obtained with a suboptimal dose of donor bone marrow or a large blood transfusion (Fig. 1). The permanent hematopoietic reconstitution of one of the cardiac recipients and prolongation of survival of four of the five others suggests that stem cells are present in other organs besides the liver but in smaller numbers.
Fig. 1.
Survival (days) of adult Lewis (LEW) rats after lethal irradiation (9.5 Gy) and syngeneic organ or bone marrow transplantation. The different numbers of unfractionated LEW bone marrow cells (0.5, 1.0, or 5.0 × 106) were used to identify the minimum number of bone marrow cells necessary for reconstitution. Three milliliters of blood is 20–25% of the rat blood volume.
Experiments of such simplicity and power have not been performed previously, presumably reflecting the entrenched belief that the hematolymphopoietic stem cells of adults require a bone marrow microenvironment to survive. This dogma came under question when it was discovered in 1992 that small numbers of donor leukocytes were present in the skin, lymph nodes, blood, and other locations of patients whose kidney or liver allografts had been functioning for up to 30 years5, 6. The implication was that donor stem cells present in the transplanted organs had migrated and survived in the recipient. Although the most easily demonstrable donor leukocytes in organ transplant recipients are dendritic cells (DCs), the presence of multiple lineages of donor origin has been confirmed after liver transplantation in both rats7 and mice8. After conventional clinical bone marrow transplantation, the mirror-image situation often arises when a trace population of host stem cells survives and persists despite patient preconditioning with supralethal cytoablative therapy9.
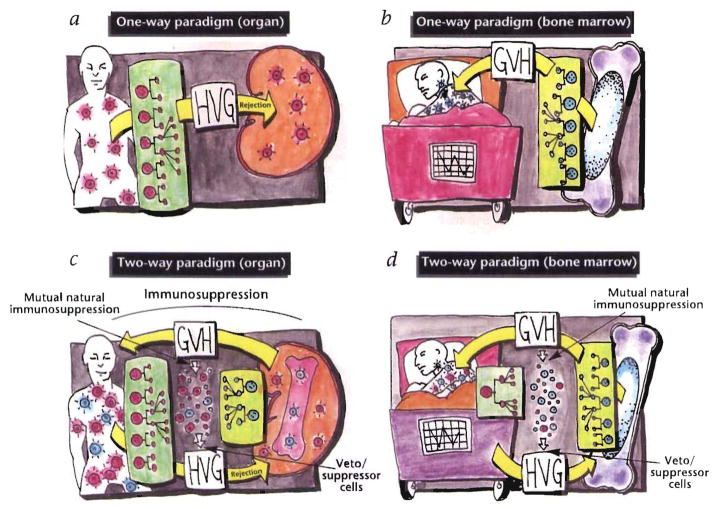
The experiments of Taniguchi et al. and Murase et al. have added substantial weight to the argument that the difference between the chimerism produced by classical bone marrow transplantation and that produced by the migrating stem cells from transplant organs is purely semantic. Following either conventional organ (Fig. 2a) or bone marrow transplantation (Fig. 2b), the quantitative disproportion of the coexisting donor and recipient leukocytes is enormous. Nevertheless, both circumstantial and direct evidence shows that the two cell populations reciprocally modulate immune responsiveness, including the induction of mutual nonreactivity (the two-way paradigm; Fig. 2c, d). This canceling effect has been postulated to explain the poor prognostic value of HLA matching before whole-organ transplantation, the rarity of GVHD after organ allografting procedures (including bowel and/or liver transplantation), and the characteristic cycle of immunologic crisis and resolution that is reflected in the clinical course of all organ recipients5, 6.
Fig. 2.
upper panels, One-way paradigm in which transplantation is conceived as involving a unidirectional immune reaction: host-versus-graft (HVG) with whole-organ transplants (a) and graft-versus-host (GVH) with bone marrow transplants (b). lower panels, Two-way paradigm in which transplantation is seen as a bidirectional and mutually canceling immune reaction that is predominantly HVG with whole-organ grafts (c), and predominantly GVH with bone marrow grafts (d).
The evidence that has accumulated since 1992 regarding leukocyte chimerism in organ recipients has revealed a connection between the acceptance of organ allografts, and the acquired neonatal tolerance described in 1953 by Billingham, Brent, and Medawar10. During the ensuing four decades, transplantation and tolerance have been defined largely in terms of a unidirectional immune reaction: host-versus-graft (HVG) following organ transplantation (Fig. 2a) and graft-versus-host (GVH) after bone marrow transplantation (Fig. 2b), Overthrow of this one-way paradigm has depended in part on finding an explanation for perpetuation of the trace population of donor leukocytes found in the organ recipients. Such an explanation has now been provided by the results of Taniguchi et al. and Murase et al. As has been postulated earlier5,6, transplantation of the liver (and possibly any organ) involves, in essence, the coincidental transplantation of pluripotent bone marrow stem cells, which are capable of renewing all hematopoietic lineages.
Numerous secondary questions arise from this concept. For example, how is the donor immune system assimilated into the larger immunologic network of the recipient with progressive development of mutual nonreactivity (bidirectional tolerance)? A protective umbrella of immunosuppression is usually needed to ensure successful engraftment. However, this may be only a temporary requirement in humans6 and outbred dogs11 and indeed immunosuppression treatment may not be necessary at all in a significant percentage of pig liver recipients11 and in liver transplants between several rat strain combinations13. As first shown by Calne et al.12, stable recipients are tolerant to subsequent transplant with other donor tissues and organs. Mouse organ transplantation models have been especially valuable for studying mechanisms of tolerance, because liver allografts are spontaneously accepted with the vast majority of strain combinations and kidney and heart allografts are accepted with a few strain combinations9.
Using the mouse liver transplant model, Lu and Thomson et al.13 have already shown that the migrating donor hematopoietic stem cells from the allograft quickly develop proliferative cellular oases particularly in the bone marrow and other lymphoid organs, where presumably pluripotent and precursor stem cells of donor and recipient origin coexist at various stages of differentiation. These microniduses are thought to constitute a growth factor-rich microenvironment that is at least partly self-generated by secretions from the coexisting community of donor and host leukocytes. Paracrine factors secreted by the parenchymal cells of the donor organ (or by recipient organs) may also contribute to successful coexistence. Candidate factors include both conventional cytokines and growth factors not usually associated with hematolymphopoiesis16. Before the discovery of spontaneous chimerism, Calne et al.12, 17 proposed that the secretion of soluble class I antigens (rather than growth factors) by allograft hepatocytes was the fundamental explanation for hepatic tolerogenicity. After the recognition of chimerism, they postulated that class I molecules are essential for the engraftment of donor hematopoietic stem cells16. However, this would imply that organ tolerogenicity is a unique attribute of the liver. Instead, it is clear that the most important determinants for the successful establishment of spontaneous chimerism are the quantity and lineage profile of the leukocytes contained in the different transplanted organs, the liver being the most favorably endowed16.
A prominent donor leukocyte in both human and animal chimeric organ allograft recipients is the DC, which has been classically perceived as the most potent of the antigen-presenting cells19 and, therefore, presents an inherent barrier to successful transplantation. However, evidence also exists for DC tolerogenicity14, 15. In the experiments of Lu et al.14. mice that spontaneously accepted liver allografts were found to have precursor donor-derived DCs that were deficient in the expression of costimulatory molecules such as B7 and, therefore, were potentially tolerogenic. In the liver recipients, the precursor cells persisted indefinitely in their disseminated locations within the recipient, where they were admixed with recipient DCs undergoing the same changes. Similar events follow heart transplantation but on a much smaller scale (summarized in ref. 15, 16). One product of the mutual cell engagement has been shown by Burlingham et al.20 to be a potent donor “veto” cell population in the blood of tolerant human kidney recipients. The changes occurring at a molecular level and how such changes relate to T-cell activation or unresponsiveness are still unresolved central issues in transplantation immunology.
The clinical implications of this evolving concept of organ allograft acceptance via chimerism are obvious. The most direct application is the augmentation of natural tolerogenic events by the adjuvant administration of donor bone marrow, a strategy long advocated empirically by Monaco et al.21 long before it was realized that bone marrow cells were able to survive in the recipient. The procedure now is under extensive clinical evaluation22. In the future when and if xenotransplantation becomes a routine procedure, the guiding principle of controlled production of chimerism will be the same.
References
- 1.Taniguchi H, Toyoshima T, Fukao K, Nakauchi H. Presence of hematopoietic stem cells in the adult liver. Nature Med. 1996;2:198–203. doi: 10.1038/nm0296-198. [DOI] [PubMed] [Google Scholar]
- 2.Murase N, et al. Multilineage hematopoietic re-constitution of supralethally irradiated rats by syngeneic whole organ transplantation: With particular reference to the liver. Transplantation. 1996;61:1–3. doi: 10.1097/00007890-199601150-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hays EF, Hays DM, Golde DW. Hemopoietic stem cells in mouse liver. Exp Hematol. 1978;6:18–27. [PubMed] [Google Scholar]
- 4.Decker T, Lohmann-Matthes ML, Baccarini M. Liver-associated macrophage precursor cells proliferate under impairment of regular hemopoiesis. Eur J Immunol. 1988;18:697–703. doi: 10.1002/eji.1830180507. [DOI] [PubMed] [Google Scholar]
- 5.Starzl TE, et al. Cell migration, chimerism, and graft acceptance. Lancet. 1992;339:1579–1582. doi: 10.1016/0140-6736(92)91840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Starzl TE, et al. Cell migration and chimerism after whole-organ transplantation: The basis of graft acceptance. Hepatology. 1993;17:1127–1152. [PMC free article] [PubMed] [Google Scholar]
- 7.Demetris AJ, et al. Hematolymphoid cell trafficking, microchimerism, and GVHD reactions after liver, bone marrow, and heart transplantation. Transplantation Proc. 1993;25:3337–3344. [PMC free article] [PubMed] [Google Scholar]
- 8.Qian S, et al. Murine liver allograft transplantation: Tolerance and donor cell chimerism. Hepatology. 1994;19:916–924. doi: 10.1002/hep.1840190418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Przepiorka D, Thomas ED, Durham DM, Fisher L. Use of a probe to repeat sequence of the Y chromosome for detection of host cells in peripheral blood of bone marrow transplant recipients. Hematopathology. 1991;95:201–206. doi: 10.1093/ajcp/95.2.201. [DOI] [PubMed] [Google Scholar]
- 10.Billingham RE, Brent L, Medawar PB. “Actively acquired tolerance” of foreign cells. Nature. 1953;172:603–606. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- 11.Starzl TE, et al. Factors determining short- and long-term survival after orthotopic liver homo-transplantation in the dog. Surgery. 1965;58:131–155. [PMC free article] [PubMed] [Google Scholar]
- 12.Calne RY. Induction of immunological tolerance by porcine liver allografts. Nature. 1969;223:472–474. doi: 10.1038/223472a0. [DOI] [PubMed] [Google Scholar]
- 13.Kamada N, Davies HffS, Roser B. Reversal of transplantation immunity by liver grafting. Nature. 1981;292:840–842. doi: 10.1038/292840a0. [DOI] [PubMed] [Google Scholar]
- 14.Lu L, et al. Growth of donor-derived dendritic cells from the bone marrow of murine liver allograft recipients in response to granulocyte/macrophage colony-stimulating factor. J Exp Med. 1995;182:379–387. doi: 10.1084/jem.182.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomson AW, et al. Microchimerism, dendritic cell progenitors and transplantation tolerance. Stem Cells. 1995;13:622–639. doi: 10.1002/stem.5530130607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murase N, et al. Variable chimerism, graft versus host disease, and tolerance after different kinds of cell and whole organ transplantation from Lewis to Brown-Norway rats. Transplantation. 1995;60:158–171. doi: 10.1097/00007890-199507000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davies HffS, Pollard SG, Calne RY. Soluble HLA antigens in the circulation of liver graft recipients. Transplantation. 1989;47:524–527. doi: 10.1097/00007890-198903000-00025. [DOI] [PubMed] [Google Scholar]
- 18.Calne R, Davies H. Organ graft tolerance: The liver effect. Lancet. 1994;343:67–68. doi: 10.1016/s0140-6736(94)90809-5. [DOI] [PubMed] [Google Scholar]
- 19.Steinman RM. The dendritic cell system and its role in immunogenicity. Ann Rev Immunology. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 20.Burlingham WJ. Microchimerism linked to cytotoxic T lymphocyte functional unresponsiveness (clonal anergy) in a tolerant renal transplant recipient. Transplantation. 1995;59:1147–1155. [PubMed] [Google Scholar]
- 21.Monaco AP, et al. Possible active enhancement of human cadaver renal allograft with antilymphocyte serum (ALS) and donor bone marrow: Case report of an initial attempt. Surgery. 1976;79:384–392. [PubMed] [Google Scholar]
- 22.Fontes P, et al. Bone marrow augmentation of donor cell chimerism in kidney, liver, heart, and pancreas islet transplantation. Lancet. 1994;344:151–155. doi: 10.1016/s0140-6736(94)92756-1. [DOI] [PMC free article] [PubMed] [Google Scholar]