Figure 4.
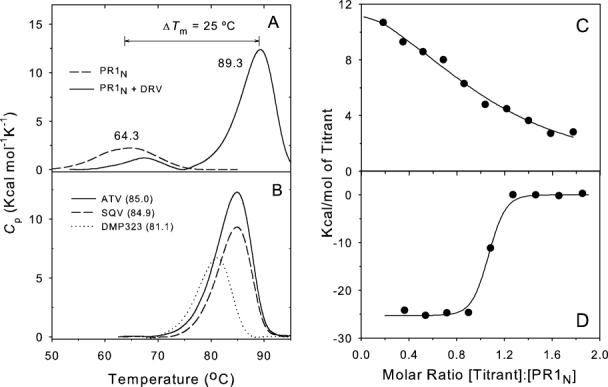
Calorimetric characterization of interaction of PR1N with inhibitors. A: DSC thermograms of mature PR1N in the absence of inhibitor and in the presence of approximately twofold molar excess of DRV. The weak thermal transition (small apparent ΔH) in the absence of inhibitor was explained by autoproteolysis during the course of the DSC experiment. B: DSC thermograms of mature PR1N in the presence of approximately twofold molar excess of the clinical inhibitors ATV (solid line) and SQV (dashed line) and the symmetrical inhibitor DMP323 (dotted line). Tm values (°C) are in parentheses. C: Isothermal titration calorimetry of PR1N (4.4 μM) in 25 mM Tris buffer, pH 7.0, containing 50 mM NaCl, at 28°C with 40 μM acetyl pepstatin. The KA determined for acetyl pepstatin is (5.6 ± 1.8) × 105M−1. D: Displacement titration of the PR1N-acetyl pepstatin complex with 40 μM DRV in the presence of 150 μM acetyl pepstatin gives an apparent binding constant ≥ 1.0 × 108M−1 for DRV, from which the KA for DRV in the absence of acetyl pepstatin is estimated to be ≥ 8 × 109M−1 (see text).
