Figure 8.
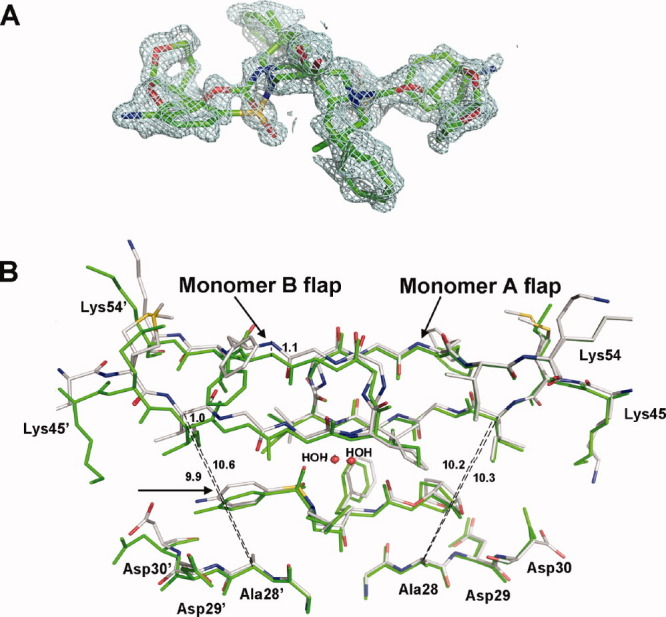
A: Electron density map of DRV in PR1N/DRV complex. The 2Fo − Fc map was contoured at 1σ level. The DRV molecule binds PR1N in two alternative orientations related by 180° with 50% occupancy each. B: Superposition of DRV binding in orientation B at the active site of PR1N/DRV and PR1M/DRV complexes. The PR1N residues are colored by element type, whereas PR1M residues are colored green. An arrow indicates the aminophenyl moiety in DRV. The flap of monomer B in PR1N/DRV is shifted by 1 Å, which increases the size of the S2′ pocket as measured by the distance between Cαs of Ala28′ and Ile47′. The shift in the position of aminophenyl moiety is in agreement with the increased size of the S2′ pocket.
