Table 1.
Optimization of enantioselective additions to sulfonylmethyl indoles.
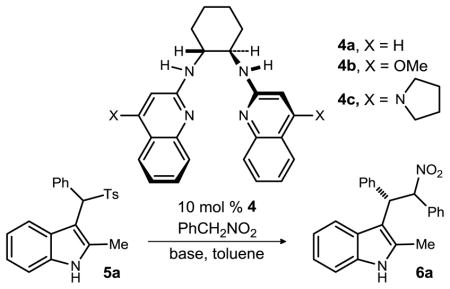 | |||||
|---|---|---|---|---|---|
| entry | catalyst | base | t(°C) | M | ee (%)b |
| 1c | 4c | KF/Alumina | rt | 0.2 | 20 |
| 2 | 4c | KF/Alumina | rt | 0.2 | 60 |
| 3 | 4c | KF/Alumina | −20 | 0.2 | 57 |
| 4 | 4c | KF/Alumina | −78 | 0.2 | 49 |
| 5 | 4c | KF | rt | 0.1 | 68 |
| 6 | 4c | Na2CO3 | rt | 0.1 | 61 |
| 7 | 4c | K2CO3 | rt | 0.2 | 70 |
| 8 | 4a | K2CO3 | rt | 0.2 | 56 |
| 9d | 4b | K2CO3 | rt | 0.2 | 73 |
| 10 | 4c | K2CO3 | rt | 0.1 | 78 |
| 11d | 4c | K2CO3 | rt | 0.1 | 81 |
All reactions were performed using 2 equivalents of phenylnitromethane on a 0.1 mmol scale and resulted in less than 1.5:1 dr material and between 30–80% yield.
Enantiomeric ratios were measured using chiral stationary phase HPLC and are reported for the major diastereomer.
Dichloromethane was used instead of toluene.
1 equivalent of phenylnitromethane was used.
Absolute stereochemistry assigned by correlation. See Supporting Information for details.
