Table 2.
Chiral base-catalyzed enantioselective nitroalkane alkylations.
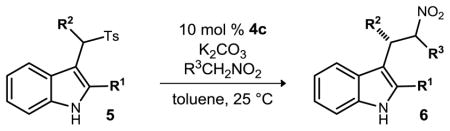 | ||||||
|---|---|---|---|---|---|---|
| entry | R1 | R2 | R3 | ee (%)b | yield(%)c (conv) | |
| 1 | Me | Ph | Ph | a | 81/81 | 78(85) |
| 2 | Me | pBrC6H4 | Ph | b | 82/75 | 74(76) |
| 3 | Me | pMeOC6H4 | Ph | c | 81/81 | 68(88) |
| 4 | Me | pF3CC6H4 | Ph | d | 84/74 | 71(78) |
| 5e | Me | oMePh | Ph | e | 76/70 | 76(95) |
| 6 | Me | 2Furyl | Ph | f | 53/44 | 69(99) |
| 7 | Me | nBu | Ph | g | 76/74 | 51(56) |
| 8d | Me | CO2Me | Ph | h | 66/47 | 47(52) |
| 9d,e | Me | CO2tBu | Ph | i | 65/66 | 55(70) |
| 10 | H | Ph | Ph | j | 40/0 | 65(68) |
| 11e | Ph | Ph | Ph | k | 74/72 | 49(52) |
| 12e | 2,5-Me-Pyrrole | Ph | Ph | l | 72/69 | 69(79) |
| 13 | Me | Ph | pMeOC6H4 | m | 89/85 | 52(77) |
| 14 | Me | Ph | pO2NC6H4 | n | 37/43 | 63(86) |
| 15e,f | Me | Ph | Me | o | 66/67 | 65(87) |
All reactions were performed on a 0.1 mmol scale using a standard 22 h reaction time and 7 equivalents of K2CO3. Diastereomeric ratios observed for crude reaction mixtures were measured by 1H NMR spectroscopy and ranged from 1:1 to 1.5:1 dr. See Supporting Information for complete details.
Enantiomeric ratios were measured using chiral stationary phase HPLC.
Isolated yields with conversions in parentheses.
3:1 dr observed.
72 h reaction time.
1.5 equivalents of nitroethane used.
