Abstract
Defects in cellular energy metabolism represent an early feature in a variety of human neurodegenerative diseases. Recent studies have shown that targeting energy metabolism can protect against neuronal cell death in such diseases. Here, we show that meclizine, a clinically used drug that we have recently shown to silence oxidative metabolism, suppresses apoptotic cell death in a murine cellular model of polyglutamine (polyQ) toxicity. We further show that this protective effect extends to neuronal dystrophy and cell death in Caenorhabditis elegans and Drosophila melanogaster models of polyQ toxicity. Meclizine's mechanism of action is not attributable to its anti-histaminergic or anti-muscarinic activity, but rather, strongly correlates with its ability to suppress mitochondrial respiration. Since meclizine is an approved drug that crosses the blood–brain barrier, it may hold therapeutic potential in the treatment of polyQ toxicity disorders, such as Huntington's disease.
INTRODUCTION
Huntington's disease (HD) is an autosomal-dominant neurodegenerative disorder characterized by progressive deterioration of cognitive and motor function (chorea) in affected individuals. HD is caused by a polyglutamine (polyQ) expansion in the huntingtin protein that leads to selective loss of striatal neurons (1). The pathological hallmarks of HD include wide-spread cortical loss and shrinkage of the brain in addition to striking neurodegeneration of the corpus striatum. Age of onset of HD is correlated with the length of the polyQ expansion (2,3). Although the HD gene was identified almost 18 years ago (4) and subsequent genetic studies in humans and model systems argue for a deleterious gain of function due to the mutation (5,6), the precise mode of HD pathogenesis remains a mystery. Several mechanisms have been proposed to explain the eventual toxicity of the mutant protein, including abnormalities in energy metabolism and oxidative damage in affected neurons (7). At present, there are no curative therapies available, only symptomatic treatments exist.
Numerous studies have suggested that the polyQ-expanded protein may alter mitochondrial energy metabolism (reviewed in 8). Indeed, a decline in the cellular ATP/ADP ratio has been strongly correlated with the length of the huntingtin polyQ region (9). Recent genetic association studies have implicated polymorphisms in mitochondrial DNA and PGC-1α as genetic modifiers for the age of onset of HD, supporting the idea that energy metabolism dysregulation is a key component of HD pathogenesis (10,11). In addition, biochemical studies in HD post-mortem tissues have shown reduced activities of pyruvate dehydrogenase (12) and respiratory complexes II, III and IV (13,14). However, respiratory chain enzyme activities are unchanged in pre-symptomatic and early-stage HD patients, suggesting that changes observed in post-mortem tissues may be secondary to the pathogenic process (15). Thus, it is not clear if diminished mitochondrial function is a primary effect of the HD mutation or is secondary to other cellular energetic defects in HD pathogenesis.
Interestingly, aberrant glycolysis has been observed in HD patients. For example, positron emission tomography studies revealed reduced glucose metabolism in cerebral cortex and basal ganglia of symptomatic HD patients (16,17). More compelling evidence for a potential causative role of reduced glucose metabolism comes from the findings that striatal hypometabolism precedes tissue loss and occurs in asymptomatic subjects (18–20). Again, the exact mechanism of reduced glucose uptake is not clear but decreased glyceraldehyde 3-phosphate dehydrogenase activity has been observed in HD patient fibroblasts (21). Furthermore, unbiased expression profiling of polyQ-huntingtin expressing cells suggested that the polyQ region in huntingtin may modulate energy metabolism via an extra-mitochondrial pathway (22), suggesting that the primary energetic defects in HD are not mitochondrial and boosting non-mitochondrial energetic pathways may offer therapeutic opportunities.
Recently, Varma et al. (23) uncovered a number of inhibitors of energy metabolism in a small molecule screen searching for suppressors of striatal cell death. The inhibitors of mitochondrial respiration were found to activate cellular survival pathways and abrogate cell death in striatal cells expressing expanded polyQ (23). However, the therapeutic potential of the compounds identified in the screen was limited by their narrow therapeutic index.
We recently devised a small molecule screening strategy to identify clinically useful drugs that are capable of shifting cellular energy metabolism from respiration to glycolysis (24). Our screen identified meclizine, a commonly used anti-emetic drug known to cross the blood–brain barrier, as an agent capable of inducing such a shift in a dose-dependent manner. We showed that meclizine confers protection against ischemia reperfusion injury in models of myocardial infarction and stroke (24). Here, we report that meclizine also confers protection against cell death and dystrophy in three different models of polyQ-related toxicity.
RESULTS
Meclizine protects striatal cells expressing polyQ-expanded huntingtin from serum withdrawal-induced apoptosis
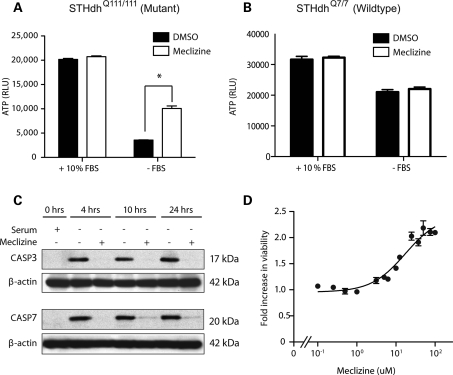
We tested meclizine in conditionally immortalized striatal cells obtained from HdhCAG knock-in mouse embryos that express endogenous levels of huntingtin with 7 (STHdhQ7/7) (wild-type) or 111(STHdhQ111/111) (mutant) glutamine residues (25). STHdhQ111/111 cells exhibit rapid death following serum withdrawal (Fig. 1A) that is much more pronounced than wild-type cells (Fig. 1B). Meclizine significantly increased cell survival in STHdhQ111/111 cells at 24 h after the removal of serum (Fig. 1A), evidently by suppressing apoptosis, based on caspase 3 and 7 cleavage (Fig. 1C). The rescue was dose-dependent with an EC50 of 17.3 µm, and a maximum efficacy of 218% increased survival over vehicle (Fig. 1D).
Figure 1.
Meclizine protects cells against serum withdrawal-induced apoptosis in mutant huntingtin expressing striatal neurons. (A and B) Cell viability measured by the ATP levels of mutant (STHdhQ111/111) and wild-type (STHdhQ7/7) striatal cells cultured in the presence or the absence of fetal bovine serum (FBS) and co-treatment with 50 µm meclizine for 24 h (n = 3, *P < 0.01). (C) Western blot analysis of protein extract from mutant STHdhQ111/111 striatal cells at three time points after the removal of serum and exposure to DMSO or 50 µm meclizine. (D) Dose–response curve showing fold change in viability of mutant STHdhQ111/111 striatal cells in serum-free media upon treatment with different concentrations of meclizine. Viability was determined by the CellTiter-Glo assay and is expressed as fold change relative to DMSO-treated cells. Solid line represents non-linear regression. Data expressed as the mean ± SD (n = 3 per data point).
Meclizine's protective effects are not due to its anti-muscarinic or anti-histaminergic activities
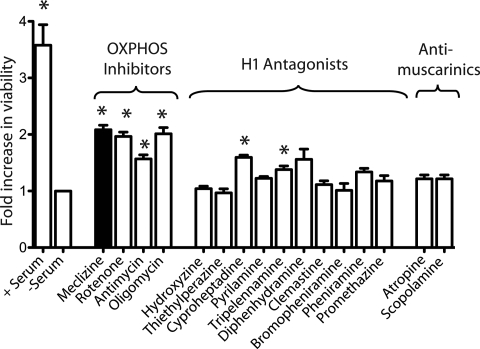
Because meclizine has multiple potential actions in the cell, we sought to determine whether the drug's anti-histamine, anti-muscarinic or anti-oxidative phosphorylation (OXPHOS) properties were responsible for protection in this cellular model. We tested 10 anti-histamines from 6 different classes including ethylenediamine (pyrilamine and tripelennamine), ethanolamine (diphenhydramine and clemastine), alkylamine (pheniramine and bromopheniramine), phenothiazine (promethazine), piperazine (hydroxyzine and thiethylperazine) and piperadine (cyproheptadine), as well as two classic anti-muscarinic compounds and three established inhibitors of OXPHOS. We tested each compound at 3–4 different doses and report the maximum efficacy achieved for each. We find that meclizine confers the most protection of all the compounds tested, and as a class, OXPHOS inhibitors provide the most protection from serum withdrawal-induced death (Fig. 2). These results are consistent with our previously published studies in heart attack and stroke models in which the protective effects of meclizine were due to its ability to attenuate mitochondrial respiration and not attributable to the blockade of the histamine or muscarinic receptor.
Figure 2.
OXPHOS inhibitors and not anti-histaminergics or anti-muscarinics confer protection against serum withdrawal-induced cell death in striatal neurons. Fold change in viability for drug treatments versus DMSO. Compounds are organized by the pharmacologic target. Serum treatment was used as a positive control. Data expressed as the mean ± SD (n = 3 per drug), *P < 0.01 after the Bonferroni correction.
Meclizine's protective effects are correlated with its inhibition of respiration
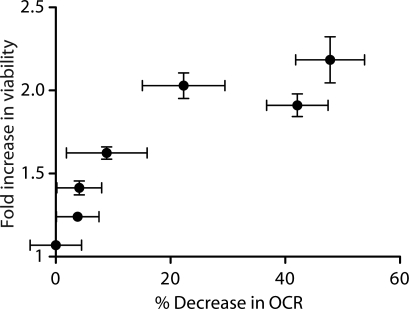
We observed a monotonic relationship in the dose response between meclizine's ability to attenuate oxygen consumption in intact striatal cells and its ability to confer increased survival to cells expressing the mutant HdhCAG allele (Fig. 3). It is notable that the survival curve as a function of inhibition of respiration is very steep, as little as a 10% decrease in the oxygen consumption rate (OCR) leads to an almost 60% increase in viability (Fig. 3). Together, these data are consistent with the notion that meclizine's protective effect on murine striatal cells expressing polyQ-expanded huntingtin is due to its ability to attenuate mitochondrial respiration.
Figure 3.
Meclizine rescue of STHdhQ111/111 viability is correlated with its inhibition of cellular respiration. The fold change in viability of STHdhQ111/111 cells and the corresponding decrease in OCR for increasing concentrations (0, 5, 10, 12.5, 25, 37.5 and 50 µm) of meclizine are plotted. Data are expressed as the mean ± SD (n ≥ 3).
Meclizine is neuroprotective in a Caenorhabditis elegans model of polyQ toxicity
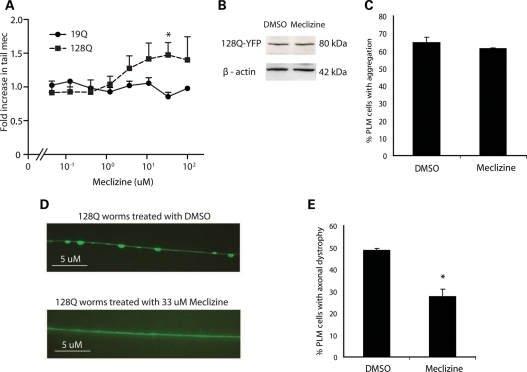
Next, we carried out blinded studies in a C. elegans model of polyQ toxicity. Caenorhabditis elegans transgenic animals expressing an N-terminal fragment of huntingtin with 128 glutamines in touch receptor neurons show neuronal dysfunction resulting in defective posterior mechanosensation (26). These animals recapitulate the early phases of expanded polyQ neurocytotoxicity in vivo, as they show neuronal dysfunction without cell death (26). As shown in Figure 4A, meclizine improved the sensory response to tail pokes in a dose-dependent manner in mutant polyQ animals, whereas no effect was detected in control animals. We observed an optimal response at a concentration of 33.3 µm, comparable with the level of drug used with murine STHdhQ111/111 cells (Fig. 1D). At this dose, we did not see a change in transgenic protein expression (Fig. 4B), and we did not observe any effect on polyQ protein aggregation (Fig. 4C). However, axonal swelling was significantly reduced (Fig. 4D and E), consistent with previous reports demonstrating that axonal dystrophy is more highly correlated with neuronal dysfunction than is the presence or the absence of polyQ aggregates (26,27).
Figure 4.
Meclizine protects against neuronal dystrophy in a worm model of polyQ toxicity. (A) Dose–response curves for the rescue of tail mechanosensation (Mec) by increasing dose of meclizine in worms expressing 19Q or 128Q repeat containing N-terminal htt proteins. Data expressed as fold change in tail Mec compared with DMSO treatment. Each data point represents the mean ± SE (n = 3, *P < 0.05). (B) Western blot analysis of protein extract from htt-Q128 expressing worms treated with DMSO or 33 µm meclizine. (C) The htt-Q128 aggregate formation was detected by light microscopy following 33 µm meclizine treatments. At least 180 PLM neurons were scored in 90 worms (n = 3). (D) Fluorescent images of the effect of DMSO or 33 µm meclizine on axonal swelling of htt-128Q expressing worms. Scale bars indicate 5 µm. (E) Axonal swelling in PLM neurons of htt-Q128 expressing worms is observed by light microscopy following DMSO or 33 µm meclizine treatments. At least 180 PLM neurons were scored in 90 worms (n = 3, *P < 0.01).
Meclizine is neuroprotective in a Drosophila melanogaster model of polyQ toxicity
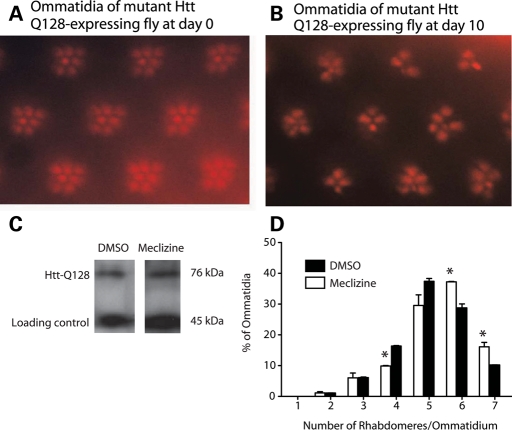
To complement the worm studies, we also tested meclizine in a Drosophila HD model in which an amino-terminal segment of 548 amino acids of human htt containing 128 glutamines is expressed in the Drosophila eye (28). Expression of the mutant N-548-htt-Q128 transgene causes a progressive decrease in the number of rhabdomeres per ommatidium (Fig. 5A and B). Treatment with 33 µm meclizine did not influence transgenic protein expression (Fig. 5C), but did protect against rhabdomere loss compared with DMSO-treated controls (Fig. 5D). Together, these C. elegans and D. melanogaster studies demonstrate that meclizine is able to suppress polyQ toxicity in vivo.
Figure 5.
Meclizine rescues photoreceptor loss in a fly model of polyQ toxicity. (A and B) Transgenic human N-548-Htt-Q128 transgene driven in photoreceptors using elav-GAL4 resulted in time-dependent neurodegeneration as assessed by the number of visible rhabdomeres. (A) Flies expressing mutant N-548-Htt-Q128 have normal complement of seven visible light collecting units (rhabdomeres) per ommatidium at day 0 post-eclosion. (B) Number of rhabdomeres at day 10 post-eclosion. (C) Western blot analysis of lysates from elav-GAL > UAS-N-548-Htt-Q128 adult flies that had been fed on either 33 µm meclizine or DMSO for 10 days. (D) Flies were treated with DMSO vehicle or meclizine at 33 µm for 10 days and the average number of rhabdomeres per ommatidium was calculated and is shown as a distribution. Data expressed as the mean ± SD (n = 2, *P < 0.05).
DISCUSSION
We previously reported that meclizine, a commonly used drug for the treatment of nausea and vertigo, is strongly cytoprotective against ischemic injury in the brain and heart (24). Since meclizine is available over-the-counter, has been used safely for over 50 years and crosses the blood–brain barrier, we sought to determine if meclizine might be protective in the context of neurodegeneration. In the current study, we tested the efficacy of meclizine in three different polyQ-expressing model systems. We show that meclizine's ability to attenuate mitochondrial respiration correlates with its ability to protect mutant huntingtin expressing striatal cells from serum withdrawal-induced apoptosis. In addition, we show that meclizine confers protection in C. elegans and D. melanogaster models of polyQ toxicity.
How could attenuating mitochondrial respiration be protective to cells? At least two possibilities exist. First, there is mounting evidence that mutated gene products in HD, Alzheimer's disease, Parkinson's disease and amyotrophic lateral sclerosis might physically interact with components of the electron transport chain (ETC) and lead to altered mitochondrial calcium handling, oxidative damage and apoptotic cell death (29–31). Thus, increased reliance on the mitochondrial respiratory chain for energy production may exacerbate the toxicity of the mutant protein. Under this hypothesis, attenuation of ETC activity provides a potential means of reducing disease pathology, as has been suggested by chemical and genetic studies (23,30,31). Second, previous studies have shown that redirecting energy metabolism toward glycolysis can suppress apoptosis (32,33). Interestingly, caspase activation has been observed in brains of patients with HD and in murine models (34,35), and apoptotic cascades have been proposed as possible targets for inhibiting cell death in HD (36,37). We have shown that meclizine's impact on energy metabolism is accompanied by a strong suppression of caspase 3 and 7 activation (Fig. 1C).
Our data suggest that meclizine-mediated protection of cells expressing mutant huntingtin is independent of the muscarinic and histamine receptors (Fig. 2), as drugs affecting these two receptors did not influence energy metabolism (24). Meclizine has been high-scoring in other recent chemical screens for compounds that diminish endogenous reactive oxygen species (38), protect against exogenous oxidative damage (39) or prevent the release of cytochrome c from mitochondria (40). Thus, we cannot rule out the possibility that meclizine might be targeting multiple mitochondrial pathways.
Meclizine has been used for decades in humans to treat nausea and vertigo and is well tolerated, with the primary side effect being drowsiness. In the current study, we have demonstrated that meclizine can shift energy metabolism with concomitant protection against polyQ toxicity. It is notable that even a small decrease in respiration corresponds to a high level of neuroprotection, reflected by the steep relationship of survival versus respiratory inhibition (Fig. 3). Given that meclizine can be titrated to achieve subtle inhibition of respiration (24), it may be possible to realize significant neuroprotection in vivo in mammals. Future studies will be required to carefully evaluate the therapeutic potential of meclizine in different murine models of polyQ toxicity. If such studies show promise at well-tolerated doses, they may warrant human trials aimed at evaluating the therapeutic potential of meclizine in a variety of neurodegenerative disorders.
MATERIALS AND METHODS
Cell culture
The murine striatal cells expressing wild-type (STHdhQ7/7) or mutant (STHdhQ111/111) huntingtin protein were grown in a DMEM high-glucose medium with 10% fetal bovine serum (Sigma Cat No. 12306C), 1× penicillin, streptomycin and glutamine and 400 μg/ml of G418 at 33°C and 5% CO2 (25).
Cell viability assays
The total cellular ATP level was measured using the CellTiter-Glo Luminescent Viability assay (Promega, G7571). Mouse striatal cells were grown for 24 h in 96-well plates at 20 000 cells/well followed by 24 h of drug treatment. Wherever indicated, cells were grown in serum-free media in the presence of a drug before ATP measurement. Luminescence was measured in a DTX880 multimode detector. Multiple concentrations (between 100 and 0.5 μm) of rotenone (Sigma), antimycin (Sigma), oligomycin (Sigma), meclizine, hydroxyzine (Spectrum Chemical), thiethylperazine (Prestwick), cyproheptadine (Sigma), pyrilamine (Spectrum Chemical), tripelennamine (Spectrum Chemical), diphenhydramine (Spectrum Chemical), clemastine (Prestwick), bromopheniramine (Spectrum Chemical), pheniramine (Spectrum Chemical), promethazine (MSDiscovery 01500510), atropine (Sigma) and scopolamine (Sigma) were tested individually for their protective effect on STHdhQ111/111 cells in serum-free media.
Measurement of cellular OCR
OCR measurements were carried out as described previously (41) with minor modifications. Wild-type STHdhQ7/7 mouse striatal cells were grown in XF24 plates at 40 000 cells/well for 20 h in their normal growth media. The assay medium was the same as the growth medium. The OCR measurements were performed every 6 min with a 2 min mixing and 2 min waiting period. Three baseline measurements were recorded prior to the addition of meclizine dihydrochloride (MP Biomedical 155341). A 50 mm stock solution of meclizine was made in DMSO, which was diluted to the specified concentration in the assay medium and its pH was adjusted to 7.4 by 1 N sodium hydroxide solution. The measurements were carried out at 33°C.
Immunoblotting
Murine STHQ111/111 striatal cells expressing mutant Htt were grown to 75% confluency in 15 cm cell culture plates. Serum-containing media was replaced with serum-free media containing either 0.1% DMSO or 50 μm meclizine. Protein extraction was performed at 0, 4, 10 and 24 h post-serum withdrawal and drug treatment using cell lysis buffer (Cell Signaling Cat. No. 9803). Protein concentration was determined using the BCA protein assay kit (Thermo Scientific Cat No. 23227). SDS–PAGE was performed using NuPAGE 4–12% Bis–Tris gel from Invitrogen (NP0321). Fifteen micrograms of protein was loaded per lane. Western blotting was performed as per the standard procedures. Cleaved caspase 3 (Cat. No. 9661) and cleaved caspase 7 (Cat. No. 9491) antibodies were purchased from Cell Signaling. β-Actin antibody was purchased from Sigma (A1978).
Drug testing in C. elegans
Animals co-expressing YFP and N-terminal htt fused to CFP in touch receptor neurons were used for drug testing (26). Synchronized L1 larvae, obtained by hypochlorite extraction, were incubated with drugs in 96-well plates in 50 µl of the M9 medium with OP-50 bacteria and 30 mg/ml of streptomycin, at 20°C for 3 days as described previously (26). Three independent assays were performed and a minimum of 100 worms were tested per dose. Animals were considered as touch responsive if they reacted after light touch (backward movement). Out of three touches, two or three reactions were regarded as responsive, and 0 or 1 reaction was regarded as unresponsive. htt aggregation and axonal morphology in PLM neurons were scored using light microscopy as described previously (26). htt aggregation was scored using CFP fluorescence and axonal dystrophy was quantified using YFP fluorescence to detect axonal swelling. Worms were treated with DMSO or 33 µm meclizine for 3 days. Proteins were extracted using the WormBook method, separated on NUPAGE 3–8% tris-acetate gels (Invitrogen), transferred to a membrane and the GFP-tagged 128Q-htt fragment was probed using anti-GFP antibody (Chemicon MAB2510, Temecula, CA, USA). Protein quantity was normalized using an anti-actin antibody (Millipore 691002).
Drug testing in D. melanogaster
The UAS-Htt-Q0 and UAS-Htt-Q128 lines encoding the first 548 amino acids of human Htt containing either 0 or 128 glutamines (42) were gifts from Troy Littleton. The elav-GAL4 driver was from the Bloomington Stock Center (#8765). The UAS-Htt-Q128 line was crossed to elav-GAL4/CyO to obtain UAS-Htt-Q128/elav-GAL4 flies. These adults showed no discernable degeneration by pseudopupil analysis upon eclosion, but the rhabdomeres underwent progressive degeneration over the following 10 days. In contrast, UAS-Htt-Q0 driven by elav-GAL4 did not show degeneration during this time. Equal numbers of newly eclosed UAS-Htt-Q128/elav-GAL4 flies were added to vials containing Carolina Biological Instant Fly food that had been freshly made up with water and meclizine or DMSO vehicle at different concentrations (100, 33, 11 or 3 μm). Flies were given fresh food and drug every 2 days and were maintained at 25°C throughout the experiment. Neurodegeneration was assessed using the pseudopupil technique (28) at day 10 by scoring the rhabdomere number/ommatidium from at least 8 animals for each condition, with at least 40 ommatidia being scored per animal. The experiment was performed twice and scoring was conducted in a blinded manner. Western blot analysis using mouse anti-human HTT (mAb2216 from Chemicon) was performed using lysates from UAS-Htt-Q128/elav-GAL4 adult flies that had been fed on either 33 µm meclizine or DMSO for 10 days.
FUNDING
This work was supported by grants from United Mitochondrial Disease Foundation (V.M.G.); Howard Hughes Medical Institute (S.A.S. and V.K.M.); Fondation pour la Recherche Médicale (N.O. and C.N.); Institut National de la Santé et de la Recherche Médicale (C.N.); the National Institutes of Health (NS32765 to M.E.M. and NS16367 to J.F.G. and M.E.M.); the Huntington's Disease Society of America Coalition for the Cure (M.E.M. and J.F.G.); the Center for Integration of Medical and Innovative Technology (V.K.M.); the Burroughs Wellcome Fund (V.K.M.) and the Smith Family Foundation (V.K.M.).
ACKNOWLEDGEMENTS
We thank Cheryl Wellington and Michael Hayden for providing the cDNA for mutant huntingtin used in the worm experiments and Lawrence Marsh, Troy Littleton and Hemant Varma for reagents and advice on the fly experiments. We thank Morgan Thompson and Casey Belcher-Timme for helpful comments on the manuscript.
Conflicts of Interest statement. V.K.M., V.M.G. and S.A.S. are listed as inventors on a patent application filed by the Massachusetts General Hospital.
REFERENCES
- 1.Vonsattel J.P., DiFiglia M. Huntington disease. J. Neuropathol. Exp. Neurol. 1998;57:369–384. doi: 10.1097/00005072-199805000-00001. doi:10.1097/00005072-199805000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Snell R.G., MacMillan J.C., Cheadle J.P., Fenton I., Lazarou L.P., Davies P., MacDonald M.E., Gusella J.F., Harper P.S., Shaw D.J. Relationship between trinucleotide repeat expansion and phenotypic variation in Huntington's disease. Nat. Genet. 1993;4:393–397. doi: 10.1038/ng0893-393. doi:10.1038/ng0893-393. [DOI] [PubMed] [Google Scholar]
- 3.Andrew S.E., Goldberg Y.P., Kremer B., Telenius H., Theilmann J., Adam S., Starr E., Squitieri F., Lin B., Kalchman M.A., et al. The relationship between trinucleotide (CAG) repeat length and clinical features of Huntington's disease. Nat. Genet. 1993;4:398–403. doi: 10.1038/ng0893-398. doi:10.1038/ng0893-398. [DOI] [PubMed] [Google Scholar]
- 4.The Huntington's Disease Collaborative Research Group. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-e. doi:10.1016/0092-8674(93)90585-E. [DOI] [PubMed] [Google Scholar]
- 5.Rubinsztein D.C. Lessons from animal models of Huntington's disease. Trends Genet. 2002;18:202–209. doi: 10.1016/s0168-9525(01)02625-7. doi:10.1016/S0168-9525(01)02625-7. [DOI] [PubMed] [Google Scholar]
- 6.Gusella J.F., MacDonald M.E. Huntington's disease: seeing the pathogenic process through a genetic lens. Trends Biochem. Sci. 2006;31:533–540. doi: 10.1016/j.tibs.2006.06.009. doi:10.1016/j.tibs.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 7.Browne S.E., Beal M.F. The energetics of Huntington's disease. Neurochem. Res. 2004;29:531–546. doi: 10.1023/b:nere.0000014824.04728.dd. doi:10.1023/B:NERE.0000014824.04728.dd. [DOI] [PubMed] [Google Scholar]
- 8.Browne S.E. Mitochondria and Huntington's disease pathogenesis: insight from genetic and chemical models. Ann. N. Y. Acad. Sci. 2008;1147:358–382. doi: 10.1196/annals.1427.018. doi:10.1196/annals.1427.018. [DOI] [PubMed] [Google Scholar]
- 9.Seong I.S., Ivanova E., Lee J.M., Choo Y.S., Fossale E., Anderson M., Gusella J.F., Laramie J.M., Myers R.H., Lesort M., et al. HD CAG repeat implicates a dominant property of huntingtin in mitochondrial energy metabolism. Hum. Mol. Genet. 2005;14:2871–2880. doi: 10.1093/hmg/ddi319. doi:10.1093/hmg/ddi319. [DOI] [PubMed] [Google Scholar]
- 10.Arning L., Haghikia A., Taherzadeh-Fard E., Saft C., Andrich J., Pula B., Hoxtermann S., Wieczorek S., Akkad D.A., Perrech M., et al. Mitochondrial haplogroup H correlates with ATP levels and age at onset in Huntington disease. J. Mol. Med. 2010;88:431–436. doi: 10.1007/s00109-010-0589-2. doi:10.1007/s00109-010-0589-2. [DOI] [PubMed] [Google Scholar]
- 11.Taherzadeh-Fard E., Saft C., Andrich J., Wieczorek S., Arning L. PGC-1alpha as modifier of onset age in Huntington disease. Mol. Neurodegener. 2009;4:10. doi: 10.1186/1750-1326-4-10. doi:10.1186/1750-1326-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butterworth J., Yates C.M., Reynolds G.P. Distribution of phosphate-activated glutaminase, succinic dehydrogenase, pyruvate dehydrogenase and gamma-glutamyl transpeptidase in post-mortem brain from Huntington's disease and agonal cases. J. Neurol. Sci. 1985;67:161–171. doi: 10.1016/0022-510x(85)90112-1. doi:10.1016/0022-510X(85)90112-1. [DOI] [PubMed] [Google Scholar]
- 13.Browne S.E., Bowling A.C., MacGarvey U., Baik M.J., Berger S.C., Muqit M.M., Bird E.D., Beal M.F. Oxidative damage and metabolic dysfunction in Huntington's disease: selective vulnerability of the basal ganglia. Ann. Neurol. 1997;41:646–653. doi: 10.1002/ana.410410514. doi:10.1002/ana.410410514. [DOI] [PubMed] [Google Scholar]
- 14.Gu M., Gash M.T., Mann V.M., Javoy-Agid F., Cooper J.M., Schapira A.H. Mitochondrial defect in Huntington's disease caudate nucleus. Ann. Neurol. 1996;39:385–389. doi: 10.1002/ana.410390317. doi:10.1002/ana.410390317. [DOI] [PubMed] [Google Scholar]
- 15.Guidetti P., Charles V., Chen E.Y., Reddy P.H., Kordower J.H., Whetsell W.O., Jr, Schwarcz R., Tagle D.A. Early degenerative changes in transgenic mice expressing mutant huntingtin involve dendritic abnormalities but no impairment of mitochondrial energy production. Exp. Neurol. 2001;169:340–350. doi: 10.1006/exnr.2000.7626. doi:10.1006/exnr.2000.7626. [DOI] [PubMed] [Google Scholar]
- 16.Kuwert T., Lange H.W., Langen K.J., Herzog H., Aulich A., Feinendegen L.E. Cortical and subcortical glucose consumption measured by PET in patients with Huntington's disease. Brain. 1990;113(Pt 5):1405–1423. doi: 10.1093/brain/113.5.1405. doi:10.1093/brain/113.5.1405. [DOI] [PubMed] [Google Scholar]
- 17.Kuhl D.E., Markham C.H., Metter E.J., Riege W.H., Phelps M.E., Mazziotta J.C. Local cerebral glucose utilization in symptomatic and presymptomatic Huntington's disease. Res. Publ. Assoc. Res. Nerv. Ment. Dis. 1985;63:199–209. [PubMed] [Google Scholar]
- 18.Grafton S.T., Mazziotta J.C., Pahl J.J., St George-Hyslop P., Haines J.L., Gusella J., Hoffman J.M., Baxter L.R., Phelps M.E. Serial changes of cerebral glucose metabolism and caudate size in persons at risk for Huntington's disease. Arch. Neurol. 1992;49:1161–1167. doi: 10.1001/archneur.1992.00530350075022. [DOI] [PubMed] [Google Scholar]
- 19.Kuwert T., Lange H.W., Boecker H., Titz H., Herzog H., Aulich A., Wang B.C., Nayak U., Feinendegen L.E. Striatal glucose consumption in chorea-free subjects at risk of Huntington's disease. J. Neurol. 1993;241:31–36. doi: 10.1007/BF00870669. doi:10.1007/BF00870669. [DOI] [PubMed] [Google Scholar]
- 20.Antonini A., Leenders K.L., Spiegel R., Meier D., Vontobel P., Weigell-Weber M., Sanchez-Pernaute R., de Yebenez J.G., Boesiger P., Weindl A., et al. Striatal glucose metabolism and dopamine D2 receptor binding in asymptomatic gene carriers and patients with Huntington's disease. Brain. 1996;119(Pt 6):2085–2095. doi: 10.1093/brain/119.6.2085. doi:10.1093/brain/119.6.2085. [DOI] [PubMed] [Google Scholar]
- 21.Mazzola J.L., Sirover M.A. Reduction of glyceraldehyde-3-phosphate dehydrogenase activity in Alzheimer's disease and in Huntington's disease fibroblasts. J. Neurochem. 2001;76:442–449. doi: 10.1046/j.1471-4159.2001.00033.x. doi:10.1046/j.1471-4159.2001.00033.x. [DOI] [PubMed] [Google Scholar]
- 22.Lee J.M., Ivanova E.V., Seong I.S., Cashorali T., Kohane I., Gusella J.F., MacDonald M.E. Unbiased gene expression analysis implicates the huntingtin polyglutamine tract in extra-mitochondrial energy metabolism. PLoS Genet. 2007;3:e135. doi: 10.1371/journal.pgen.0030135. doi:10.1371/journal.pgen.0030135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varma H., Cheng R., Voisine C., Hart A.C., Stockwell B.R. Inhibitors of metabolism rescue cell death in Huntington's disease models. Proc. Natl Acad. Sci. USA. 2007;104:14525–14530. doi: 10.1073/pnas.0704482104. doi:10.1073/pnas.0704482104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gohil V.M., Sheth S.A., Nilsson R., Wojtovich A.P., Lee J.H., Perocchi F., Chen W., Clish C.B., Ayata C., Brookes P.S., et al. Nutrient-sensitized screening for drugs that shift energy metabolism from mitochondrial respiration to glycolysis. Nat. Biotechnol. 2010;28:249–255. doi: 10.1038/nbt.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trettel F., Rigamonti D., Hilditch-Maguire P., Wheeler V.C., Sharp A.H., Persichetti F., Cattaneo E., MacDonald M.E. Dominant phenotypes produced by the HD mutation in STHdh(Q111) striatal cells. Hum. Mol. Genet. 2000;9:2799–2809. doi: 10.1093/hmg/9.19.2799. doi:10.1093/hmg/9.19.2799. [DOI] [PubMed] [Google Scholar]
- 26.Parker J.A., Connolly J.B., Wellington C., Hayden M., Dausset J., Neri C. Expanded polyglutamines in Caenorhabditis elegans cause axonal abnormalities and severe dysfunction of PLM mechanosensory neurons without cell death. Proc. Natl Acad. Sci. USA. 2001;98:13318–13323. doi: 10.1073/pnas.231476398. doi:10.1073/pnas.231476398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parker J.A., Arango M., Abderrahmane S., Lambert E., Tourette C., Catoire H., Neri C. Resveratrol rescues mutant polyglutamine cytotoxicity in nematode and mammalian neurons. Nat. Genet. 2005;37:349–350. doi: 10.1038/ng1534. doi:10.1038/ng1534. [DOI] [PubMed] [Google Scholar]
- 28.Jackson G.R., Salecker I., Dong X., Yao X., Arnheim N., Faber P.W., MacDonald M.E., Zipursky S.L. Polyglutamine-expanded human huntingtin transgenes induce degeneration of Drosophila photoreceptor neurons. Neuron. 1998;21:633–642. doi: 10.1016/s0896-6273(00)80573-5. doi:10.1016/S0896-6273(00)80573-5. [DOI] [PubMed] [Google Scholar]
- 29.Knott A.B., Perkins G., Schwarzenbacher R., Bossy-Wetzel E. Mitochondrial fragmentation in neurodegeneration. Nat. Rev. Neurosci. 2008;9:505–518. doi: 10.1038/nrn2417. doi:10.1038/nrn2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buttner S., Bitto A., Ring J., Augsten M., Zabrocki P., Eisenberg T., Jungwirth H., Hutter S., Carmona-Gutierrez D., Kroemer G., et al. Functional mitochondria are required for alpha-synuclein toxicity in aging yeast. J. Biol. Chem. 2008;283:7554–7560. doi: 10.1074/jbc.M708477200. doi:10.1074/jbc.M708477200. [DOI] [PubMed] [Google Scholar]
- 31.Fukui H., Diaz F., Garcia S., Moraes C.T. Cytochrome c oxidase deficiency in neurons decreases both oxidative stress and amyloid formation in a mouse model of Alzheimer's disease. Proc. Natl Acad. Sci. USA. 2007;104:14163–14168. doi: 10.1073/pnas.0705738104. doi:10.1073/pnas.0705738104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jeong D.W., Kim T.S., Cho I.T., Kim I.Y. Modification of glycolysis affects cell sensitivity to apoptosis induced by oxidative stress and mediated by mitochondria. Biochem. Biophys. Res. Commun. 2004;313:984–991. doi: 10.1016/j.bbrc.2003.12.033. doi:10.1016/j.bbrc.2003.12.033. [DOI] [PubMed] [Google Scholar]
- 33.Hunter A.J., Hendrikse A.S., Renan M.J. Can radiation-induced apoptosis be modulated by inhibitors of energy metabolism? Int. J. Radiat. Biol. 2007;83:105–114. doi: 10.1080/09553000601121157. doi:10.1080/09553000601121157. [DOI] [PubMed] [Google Scholar]
- 34.Ona V.O., Li M., Vonsattel J.P., Andrews L.J., Khan S.Q., Chung W.M., Frey A.S., Menon A.S., Li X.J., Stieg P.E., et al. Inhibition of caspase-1 slows disease progression in a mouse model of Huntington's disease. Nature. 1999;399:263–267. doi: 10.1038/20446. doi:10.1038/20446. [DOI] [PubMed] [Google Scholar]
- 35.Sanchez I., Xu C.J., Juo P., Kakizaka A., Blenis J., Yuan J. Caspase-8 is required for cell death induced by expanded polyglutamine repeats. Neuron. 1999;22:623–633. doi: 10.1016/s0896-6273(00)80716-3. doi:10.1016/S0896-6273(00)80716-3. [DOI] [PubMed] [Google Scholar]
- 36.Vila M., Przedborski S. Targeting programmed cell death in neurodegenerative diseases. Nat. Rev. Neurosci. 2003;4:365–375. doi: 10.1038/nrn1100. doi:10.1038/nrn1100. [DOI] [PubMed] [Google Scholar]
- 37.Pattison L.R., Kotter M.R., Fraga D., Bonelli R.M. Apoptotic cascades as possible targets for inhibiting cell death in Huntington's disease. J. Neurol. 2006;253:1137–1142. doi: 10.1007/s00415-006-0198-8. doi:10.1007/s00415-006-0198-8. [DOI] [PubMed] [Google Scholar]
- 38.Vincent A.M., Feldman E.L. Can drug screening lead to candidate therapies for testing in diabetic neuropathy? Antioxid. Redox Signal. 2008;10:387–393. doi: 10.1089/ars.2007.1815. doi:10.1089/ars.2007.1815. [DOI] [PubMed] [Google Scholar]
- 39.Sarang S.S., Yoshida T., Cadet R., Valeras A.S., Jensen R.V., Gullans S.R. Discovery of molecular mechanisms of neuroprotection using cell-based bioassays and oligonucleotide arrays. Physiol. Genomics. 2002;11:45–52. doi: 10.1152/physiolgenomics.00064.2002. [DOI] [PubMed] [Google Scholar]
- 40.Wang X., Zhu S., Pei Z., Drozda M., Stavrovskaya I.G., Del Signore S.J., Cormier K., Shimony E.M., Wang H., Ferrante R.J., et al. Inhibitors of cytochrome c release with therapeutic potential for Huntington's disease. J. Neurosci. 2008;28:9473–9485. doi: 10.1523/JNEUROSCI.1867-08.2008. doi:10.1523/JNEUROSCI.1867-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu M., Neilson A., Swift A.L., Moran R., Tamagnine J., Parslow D., Armistead S., Lemire K., Orrell J., Teich J., et al. Multiparameter metabolic analysis reveals a close link between attenuated mitochondrial bioenergetic function and enhanced glycolysis dependency in human tumor cells. Am. J. Physiol. Cell Physiol. 2007;292:C125–C136. doi: 10.1152/ajpcell.00247.2006. doi:10.1152/ajpcell.00247.2006. [DOI] [PubMed] [Google Scholar]
- 42.Lee W.C., Yoshihara M., Littleton J.T. Cytoplasmic aggregates trap polyglutamine-containing proteins and block axonal transport in a Drosophila model of Huntington's disease. Proc. Natl Acad. Sci. USA. 2004;101:3224–3229. doi: 10.1073/pnas.0400243101. doi:10.1073/pnas.0400243101. [DOI] [PMC free article] [PubMed] [Google Scholar]