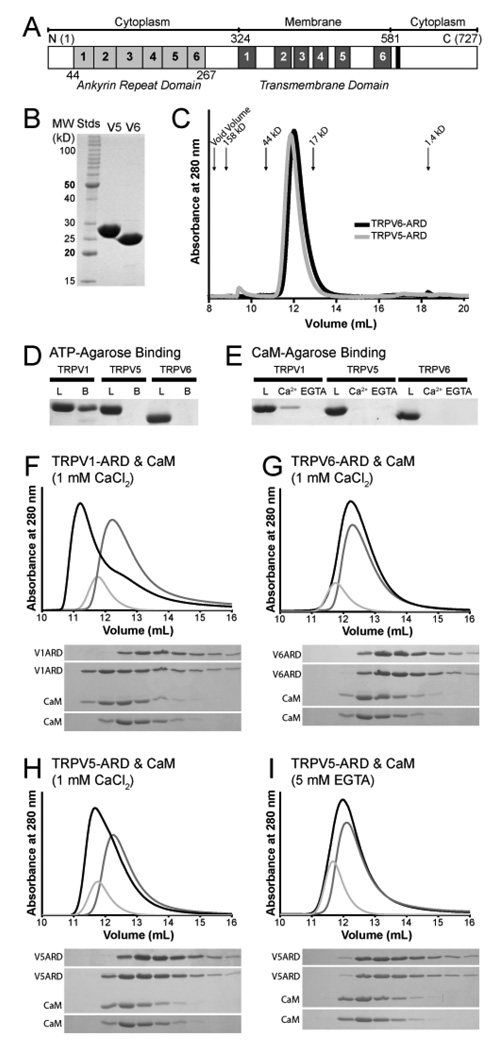
Figure 1.
Biochemical properties of the TRPV5-ARD and TRPV6-ARD. (A) Schematic representation of a TRPV protein primary structure and the location of each domain. Individual ankyrin repeats and predicted transmembrane segments are numbered and colored light grey and dark grey, respectively. The conserved TRP-box in the C-terminus is colored black. The residue numbers correspond to those of mouse TRPV6. (B) 15% SDS-PAGE analysis of the TRPV5-ARD and TRPV6-ARD purified from E. coli. (C) Analytical Superdex 75 size exclusion chromatography of the TRPV5-ARD (grey) and TRPV6-ARD (black). Absorbance at 280 nm is plotted against elution volume. The void volume and elution volume of molecular weight standards are indicated. (D) ATP-agarose pull-down assay of TRPV ARDs, with 15% SDS-PAGE analysis of the loaded (L) and ATP-agarose-bound (B) protein. (E) CaM-agarose pull-down assay of TRPV ARDs, with 15% SDS-PAGE analysis of loaded protein (L) and protein bound in the presence (Ca2+) and absence (EGTA) of calcium. TRPV1-ARD (9) is included as a positive control in panels D and E. (F–I) Analysis of CaM/ARD complex formation by Superdex 75 size exclusion chromatography. Shown are representative traces from at least two experiments for TRPV1-ARD (F), TRPV6-ARD (G), and TRPV5-ARD with Ca2+ (H) or EGTA (I). Absorbance at 280 nm is plotted against elution volume with CaM alone in light grey, TRPV-ARD in grey and CaM plus TRPV-ARD in black. Shifts in retention time were confirmed by 20% SDS-PAGE analysis of 0.5 mL fractions covering the elution volumes between 10 and 15 mL for each injection.