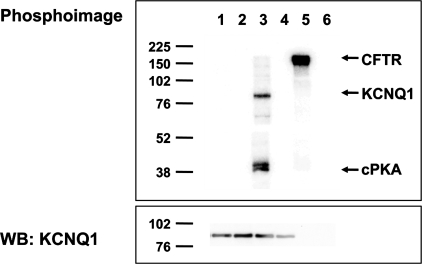
Fig. 2.
AMPK does not directly phosphorylate KCNQ1 in vitro. HEK-293T cells transfected to express either KCNQ1 (lanes 1–4) or CFTR (lanes 5 and 6) were lysed, and then the channels were immunoprecipitated from cell lysates for in vitro phosphorylation assays using either no added kinase (lanes 2, 4, and 6), purified AMPK holoenzyme (lanes 1 and 5), or purified PKA catalytic subunit (lane 3), as described in materials and methods. Representative phospho-screen (top) and Western blot (WB; bottom) images of the same nitrocellulose membrane are shown from 1 of 3 repeat experiments. Positive controls confirmed that AMPK was able to phoshorylate CFTR (lane 5), and PKA was able to phosphorylate KCNQ1 (lane 3), as previously described (34, 49). However, there was no detectable KCNQ1 phosphorylation by AMPK in vitro (lane 1). Western blot analysis confirmed comparable KCNQ1 expression in lanes 1–4 (bottom).