Abstract
Evolutionary change, whether in populations of organisms or malignant tumor cells, is contingent on the availability of inherited variation for natural selection to act upon. It is becoming clear that the Hsp90 chaperone, which normally functions to buffer client proteins against the effects of genetic variation, plays a central role in this process. Severe environmental stress can overwhelm the chaperone's buffering capacity, causing previously cryptic genetic variation to be expressed. Recent studies now indicate that in addition to exposing existing variation, Hsp90 can induce novel epigenetic and genetic changes. We discuss key findings that suggest a rich set of pathways by which Hsp90 can mediate the influences of the environment on the genome.
Keywords: Hsp90 chaperone, Stress-induced mutation, Repeat instability, Evolution
Introduction
Conrad Waddington's influential theory of genetic assimilation is a rationalization of the “inheritance of acquired characters” within the context of natural selection and Mendelian genetics. Waddington sought a non-Lamarckian explanation for how environmentally triggered traits, such as the callosities of ostriches and the hardened soles on the feet of humans, could become inherited features of an organism (Waddington 1942). He argued that morphological development must be buffered against minor environmental and genetic variations and that this canalization enhances the neutral accumulation of genetic variation. Under extreme stress, this buffering capacity is reduced, leading to the expression of latent genetic variation. Iterative rounds of selection can “assimilate” the adaptive phenotypes such that their expression gains independence from the original environmental stimulus (Waddington 1942). Waddington supported his model in Drosophila by applying stress, in the form of heat shock or ether vapor, to developing flies. He demonstrated that (1) morphological defects arise as a result of environmental stress, (2) the proportion of flies exhibiting these phenotypes increases under selection, and (3) continued selection for the novel traits results in their persistence, even in the absence of the original environmental stress (Waddington 1953; Waddington 1956). While these results were provocative, it remained unclear whether adaptive phenotypes could arise from such a process or if the novel phenotypes were simply degenerative outcomes of an inability to withstand stress (Williams 1966).
Over 50 years later, Rutherford and Lindquist (1998) reignited interest in genetic assimilation with the identification of a molecular basis for Waddington's observations. They proposed that the Hsp90 chaperone buffers cryptic genetic variation. Chaperones function to help fold and stabilize client proteins that lack intrinsically robust folding properties, buffering them against small changes that might arise in the protein sequence. In this model, the surge in misfolded client proteins accompanying certain environmental stresses, such as heat shock, overwhelms the buffering capacity of chaperones, causing previously cryptic genetic variation to be expressed (Rutherford and Lindquist 1998). Although their initial experimentation in flies produced many of the same dramatic phenotypes that Waddington observed, experiments in plants revealed that the suppression of Hsp90 produces quantitative variation in leaf and stalk morphology and life-history traits such as flowering time, without impacting viability (Queitsch et al. 2002; Sangster et al. 2007; Sangster et al. 2008). Interestingly, Hsp90 appears to modulate plant resistance to insect herbivores, further supporting a role for Hsp90 in adaptive evolution (Sangster et al. 2007).
The major prerequisite for Waddington's genetic assimilation process is the presence of latent, preexisting genetic variation segregating within the population. Ruden and colleagues challenged this assumption when they demonstrated that Hsp90 could modulate the expression and assimilation of novel morphological phenotypes in isogenic populations of flies via an epigenetic mechanism (Sollars et al. 2003). Support for an epigenetic model was further strengthened by the observation that histone deacetylase inhibitors such as trichostatin A and sodium butyrate could substantially suppress the expression of the novel phenotypes and from the regulatory role Hsp90 plays in the maintenance of active chromatin (Ruden et al. 2003; Sollars et al. 2003; Tariq et al. 2009). It is therefore likely that, for a subset of assimilated phenotypes, preexisting genetic variation is not a requirement. In addition, stress-induced epigenetic changes have the potential to be adaptive, as they allow populations to rapidly and reversibly respond to changing environments (Rando and Verstrepen 2007).
If sorting through the genetic and epigenetic contributions to stress-induced phenotypes was not complex enough, the genetic assimilation model is now being further complicated by new results that suggest Hsp90 plays a role in maintaining the integrity of the genome. First, Specchia et al. (2010) reported that impairment of the chaperone leads to transposon-mediated mutagenesis in the Drosophila germline. The authors observed the assimilation of a Scutoid-like phenotype (loss of bristles and perturbed development of the compound eye) and determined that it arose from an insertional mutagenesis event in the noc gene. The insertion generated a truncated transcription factor and was found only in the flies exhibiting the novel phenotype. Sequencing of the noc gene in wild-type flies from the experiment revealed no changes to the gene sequence. These observations offer an additional explanation for the contribution of genetic background to the expression of stress-induced phenotypes, as different genetic backgrounds could lead to different transposon insertion events (Specchia et al. 2010).
Most recently, we have demonstrated that Hsp90 is required for maintaining microsatellite repeat stability in human cells, through the regulation of double-strand break (DSB) repair (Mittelman et al. 2010). In mammalian cells, DSBs are repaired by two well-defined, homology-dependent repair pathways: strand invasion, which depends on Rad51, and single-strand annealing (SSA), which does not (Paques and Haber 1999). We showed that when Hsp90 is diverted from its normal function, the levels of active Rad51 decrease, suggesting that DSB-repair shifts towards the SSA pathway. Because the SSA pathway fixes DSBs by pairing homologous sequences on either side of the break, it would lead naturally to changes in the lengths of the repeat tract (Richard et al. 1999). Therefore, pressure from the environment might play a role in directing whether repeat tracts are repaired by the conservative Rad51-dependent pathway or the more error-prone, SSA pathway. Our results are consistent with the provocative findings from Rosenberg and colleagues, who have extensively characterized stress-induced error-prone DSB-repair in bacteria (Galhardo et al. 2007; Ponder et al. 2005).
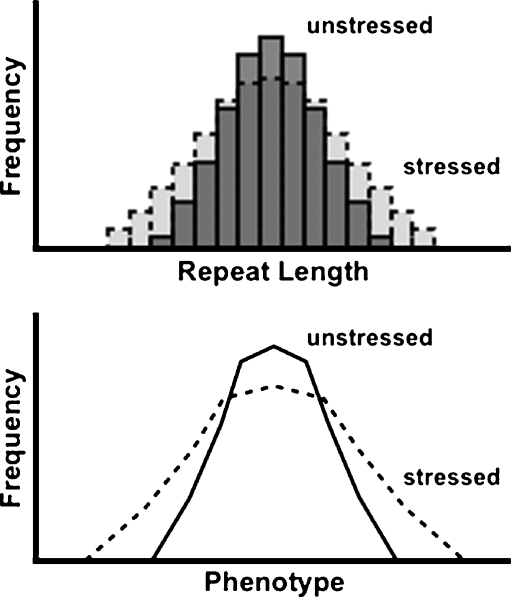
Repeat mutation has long been used as an indicator of genome stability and is of significant prognostic utility for certain types of cancer (Healy et al. 2006; Reuschenbach et al. 2009). The role of repeats as agents of disease extends beyond the two dozen or so characterized repeat expansion neurodegenerative diseases to include a wide range of neurological and morphological disorders (Albrecht and Mundlos 2005; Orr and Zoghbi 2007). This common focus on disease, however, masks the inherent utility of repeats in modulating gene function. Coding microsatellites are enriched in genes for transcription factors and other regulatory proteins, and changes in the length of these repeats exert incremental impacts on gene function (Albrecht et al. 2004; Bacolla et al. 2008; Gerber et al. 1994; Wren et al. 2000). Variations in the lengths of noncoding repeats in the promoters of genes have been shown to quantitatively affect transcription and have likely facilitated transcriptional evolution (Vinces et al. 2009). Emerging evidence implicates coding and noncoding microsatellites as important sources of common, subclinical genetic variation in morphological and behavioral traits in numerous species, including humans, dogs, and flies (Fondon and Garner 2004; Goodman et al. 1997; Kashi and King 2006; Sawyer et al. 1997). It is possible that the incremental impact of variation in intragenic repeats might provide insight into the subtle, quantitative trait variation recently described in Hsp90-supressed flies and plants (Milton et al. 2006; Queitsch et al. 2002; Sangster et al. 2007; Fig. 1).
Fig. 1.
Stress-induced repeat instability as a potential source of novel phenotypic variation. Under normal environmental conditions, repeat allele lengths are distributed narrowly in a population (dark gray bars), but when severe stress diverts Hsp90 from its normal role in maintaining genome stability, the distribution of repeat lengths broadens (light gray bars). Similarly, an unstressed population expresses a narrow range of phenotypes (solid line), which expands when stress is applied (dashed line). The ability of microsatellite repeats to incrementally influence gene expression suggests that stress-induced repeat instability may underlie some fraction of the increased range of phenotypes observed in response to stress
The identification of the Hsp90 chaperone as a buffer for latent genetic variation has transformed our understanding of Waddington's theory of genetic assimilation. Although controversy exists regarding the extent to which genetic assimilation has played a role in evolution, there are clear examples of how the suppression of Hsp90, under extreme stress, can reveal potentially adaptive phenotypes. Recent studies now indicate that in addition to exposing existing cryptic variation, Hsp90 modulates the generation of novel epigenetic and genetic variation. These results imply a rich set of pathways by which the environment can influence the genome.
If Hsp90 and stress can regulate the integrity of the genome, what does this say about the relationship between the evolutionary forces of mutation and selection? Have we historically overinterpreted the conclusions of Luria and Delbruck (1943) in asserting that selection and mutation are completely independent? Could the genome be more dynamic and responsive to the environment than we previously thought? What are the implications of a malleable genome for cancer and other human diseases? Undoubtedly, these questions will be addressed as future studies continue to explore the ramifications of a genome sensitive to its environment.
Acknowledgements
We would like to thank Dr. John W. Fondon III and Dr. Steve W. Lockless for insightful discussions and manuscript suggestions. This work was supported by an F-32 grant from the NIH (NS064762) to D.M. and an R01 grant from the NIH (GM38219) to J.H.W.
References
- Albrecht A, Mundlos S. The other trinucleotide repeat: polyalanine expansion disorders. Curr Opin Genet Dev. 2005;15:285–293. doi: 10.1016/j.gde.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Albrecht AN, Kornak U, Boddrich A, Suring K, Robinson PN, Stiege AC, Lurz R, Stricker S, Wanker EE, Mundlos S. A molecular pathogenesis for transcription factor associated poly-alanine tract expansions. Hum Mol Genet. 2004;13:2351–2359. doi: 10.1093/hmg/ddh277. [DOI] [PubMed] [Google Scholar]
- Bacolla A, Larson JE, Collins JR, Li J, Milosavljevic A, Stenson PD, Cooper DN, Wells RD. Abundance and length of simple repeats in vertebrate genomes are determined by their structural properties. Genome Res. 2008;18:1545–1553. doi: 10.1101/gr.078303.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fondon JW, 3rd, Garner HR. Molecular origins of rapid and continuous morphological evolution. Proc Natl Acad Sci USA. 2004;101:18058–18063. doi: 10.1073/pnas.0408118101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galhardo RS, Hastings PJ, Rosenberg SM. Mutation as a stress response and the regulation of evolvability. Crit Rev Biochem Mol Biol. 2007;42:399–435. doi: 10.1080/10409230701648502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber HP, Seipel K, Georgiev O, Hofferer M, Hug M, Rusconi S, Schaffner W. Transcriptional activation modulated by homopolymeric glutamine and proline stretches. Science. 1994;263:808–811. doi: 10.1126/science.8303297. [DOI] [PubMed] [Google Scholar]
- Goodman FR, Mundlos S, Muragaki Y, Donnai D, Giovannucci-Uzielli ML, Lapi E, Majewski F, McGaughran J, McKeown C, Reardon W, Upton J, Winter RM, Olsen BR, Scambler PJ. Synpolydactyly phenotypes correlate with size of expansions in HOXD13 polyalanine tract. Proc Natl Acad Sci USA. 1997;94:7458–7463. doi: 10.1073/pnas.94.14.7458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy C, Wade M, McMahon A, Williams A, Johnson DA, Parfett C. Flow cytometric detection of tandem repeat mutations induced by various chemical classes. Mutat Res. 2006;598:85–102. doi: 10.1016/j.mrfmmm.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Kashi Y, King DG. Simple sequence repeats as advantageous mutators in evolution. Trends Genet. 2006;22:253–259. doi: 10.1016/j.tig.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Luria SE, Delbruck M. Mutations of bacteria from virus sensitivity to virus resistance. Genetics. 1943;28:491–511. doi: 10.1093/genetics/28.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton CC, Ulane CM, Rutherford S. Control of canalization and evolvability by Hsp90. PLoS ONE. 2006;1:e75. doi: 10.1371/journal.pone.0000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittelman D, Sykoudis K, Hersh M, Lin Y, Wilson JH (2010) Hsp90 modulates CAG repeat instability in human cells. Cell Stress Chaperones [DOI] [PMC free article] [PubMed]
- Orr HT, Zoghbi HY. Trinucleotide repeat disorders. Annu Rev Neurosci. 2007;30:575–621. doi: 10.1146/annurev.neuro.29.051605.113042. [DOI] [PubMed] [Google Scholar]
- Paques F, Haber JE. Multiple pathways of recombination induced by double-strand breaks in Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 1999;63:349–404. doi: 10.1128/mmbr.63.2.349-404.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponder RG, Fonville NC, Rosenberg SM. A switch from high-fidelity to error-prone DNA double-strand break repair underlies stress-induced mutation. Mol Cell. 2005;19:791–804. doi: 10.1016/j.molcel.2005.07.025. [DOI] [PubMed] [Google Scholar]
- Queitsch C, Sangster TA, Lindquist S. Hsp90 as a capacitor of phenotypic variation. Nature. 2002;417:618–624. doi: 10.1038/nature749. [DOI] [PubMed] [Google Scholar]
- Rando OJ, Verstrepen KJ. Timescales of genetic and epigenetic inheritance. Cell. 2007;128:655–668. doi: 10.1016/j.cell.2007.01.023. [DOI] [PubMed] [Google Scholar]
- Reuschenbach M, Kloor M, Morak M, Wentzensen N, Germann A, Garbe Y, Tariverdian M, Findeisen P, Neumaier M, Holinski-Feder E, Knebel DM. Serum antibodies against frameshift peptides in microsatellite unstable colorectal cancer patients with Lynch syndrome. Fam Cancer. 2009;9:173–179. doi: 10.1007/s10689-009-9307-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard GF, Dujon B, Haber JE. Double-strand break repair can lead to high frequencies of deletions within short CAG/CTG trinucleotide repeats. Mol Gen Genet. 1999;261:871–882. doi: 10.1007/s004380050031. [DOI] [PubMed] [Google Scholar]
- Ruden DM, Garfinkel MD, Sollars VE, Lu X. Waddington's widget: Hsp90 and the inheritance of acquired characters. Semin Cell Dev Biol. 2003;14:301–310. doi: 10.1016/j.semcdb.2003.09.024. [DOI] [PubMed] [Google Scholar]
- Rutherford SL, Lindquist S. Hsp90 as a capacitor for morphological evolution. Nature. 1998;396:336–342. doi: 10.1038/24550. [DOI] [PubMed] [Google Scholar]
- Sangster TA, Bahrami A, Wilczek A, Watanabe E, Schellenberg K, McLellan C, Kelley A, Kong SW, Queitsch C, Lindquist S. Phenotypic diversity and altered environmental plasticity in Arabidopsis thaliana with reduced Hsp90 levels. PLoS ONE. 2007;2:e648. doi: 10.1371/journal.pone.0000648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangster TA, Salathia N, Undurraga S, Milo R, Schellenberg K, Lindquist S, Queitsch C. HSP90 affects the expression of genetic variation and developmental stability in quantitative traits. Proc Natl Acad Sci USA. 2008;105:2963–2968. doi: 10.1073/pnas.0712200105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer LA, Hennessy JM, Peixoto AA, Rosato E, Parkinson H, Costa R, Kyriacou CP. Natural variation in a Drosophila clock gene and temperature compensation. Science. 1997;278:2117–2120. doi: 10.1126/science.278.5346.2117. [DOI] [PubMed] [Google Scholar]
- Sollars V, Lu X, Xiao L, Wang X, Garfinkel MD, Ruden DM. Evidence for an epigenetic mechanism by which Hsp90 acts as a capacitor for morphological evolution. Nat Genet. 2003;33:70–74. doi: 10.1038/ng1067. [DOI] [PubMed] [Google Scholar]
- Specchia V, Piacentini L, Tritto P, Fanti L, D'Alessandro R, Palumbo G, Pimpinelli S, Bozzetti MP. Hsp90 prevents phenotypic variation by suppressing the mutagenic activity of transposons. Nature. 2010;463:662–665. doi: 10.1038/nature08739. [DOI] [PubMed] [Google Scholar]
- Tariq M, Nussbaumer U, Chen Y, Beisel C, Paro R. Trithorax requires Hsp90 for maintenance of active chromatin at sites of gene expression. Proc Natl Acad Sci USA. 2009;106:1157–1162. doi: 10.1073/pnas.0809669106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinces MD, Legendre M, Caldara M, Hagihara M, Verstrepen KJ. Unstable tandem repeats in promoters confer transcriptional evolvability. Science. 2009;324:1213–1216. doi: 10.1126/science.1170097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddington CH. Canalization of development and the inheritance of acquired characters. Nature. 1942;150:563–565. doi: 10.1038/150563a0. [DOI] [PubMed] [Google Scholar]
- Waddington CH. Genetic assimilation of an acquired character. Evolution. 1953;7:118–126. doi: 10.2307/2405747. [DOI] [Google Scholar]
- Waddington CH. Genetic assimilation of the bithorax phenotype. Evolution. 1956;10:1–13. doi: 10.2307/2406091. [DOI] [Google Scholar]
- Williams GC. Adaptation and natural selection: a critique of some current evolutionary thought. Princeton: Princeton University Press; 1966. [Google Scholar]
- Wren JD, Forgacs E, Fondon JW, 3rd, Pertsemlidis A, Cheng SY, Gallardo T, Williams RS, Shohet RV, Minna JD, Garner HR. Repeat polymorphisms within gene regions: phenotypic and evolutionary implications. Am J Hum Genet. 2000;67:345–356. doi: 10.1086/303013. [DOI] [PMC free article] [PubMed] [Google Scholar]