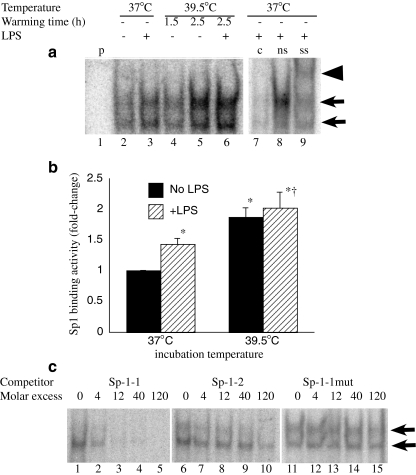
Fig. 4.
FRT enhances Sp1 activation. RAW 264.7 macrophages were prewarmed to 37°C or 39.5°C for 90 min, stimulated with 100 ng/ml LPS and incubated at the same temperature for an additional 1 h or warmed at 39.5°C for 90 or 150 min without LPS. Nuclear extracts were analyzed by EMSA for binding to a consensus Sp1 probe. A representative autoradiograph (a) and mean ± SE of EMSA band density determined by phosphorimaging from three separate experiments (b) are shown. The Sp1 binding complex appears as a doublet band indicated by arrows in a. Lane 1 is probe alone (p without cell extract). Binding specificity was confirmed by competition with unlabeled probe (c) (compare lane 7 with nonspecific (ns) oligonucleotide competition in lane 8) and Sp1 was confirmed by supershifting (ss) with an anti-Sp1 antibody (arrowhead, lane 9). c RAW 264.7 macrophages were stimulated with 100 ng/ml LPS and incubated at 37°C for 1 h and the capacity of the sequences comprising the proximal Sp1 (Sp1-1, −70/−44 nt) and distal Sp1 (Sp1-2, −184/−158 nt) as competitors was analyzed. Lanes 1–5 (cold Sp1-1 oligonucleotides), lanes 6–10 (cold Sp1-2 oligonucleotides), and lanes 11–15 (mutant Sp1-1 oligonucleotide)