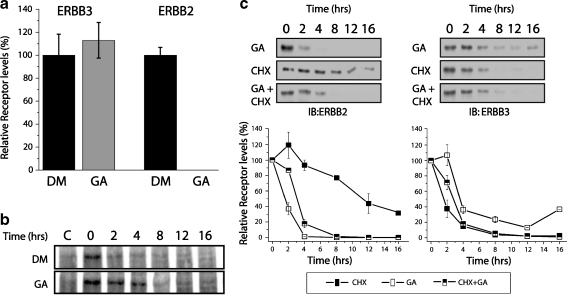
Fig. 3.
Mature ERBB3 is not destabilized by GA. a Levels of cell surface ERBB2 and ERBB3 after GA treatment, relative to unchallenged turnover. MCF7 cells were cell-surface-biotinylated with 1 mg/ml NHS-biotin and subsequently treated with 3 µM GA or DMSO (vehicle control) for 6 h prior to lysis. Biotinylated proteins were enriched on streptavidin-conjugated agarose beads and analyzed by SDS gel electrophoresis and Western blotting using antibodies against ERBB3 (C17) or ERBB2 (C18). Densitometry measurements were taken from the Western blot, and the relative changes for GA-treated samples were normalized to levels of DMSO control samples. b Turnover of 35S-labeled, mature ERBB3 is not accelerated by GA. MCF7 cells were pulse-labeled with 35S cysteine and methionine for 16 h, followed by two washes with media containing no label. Cells were allowed to recover from washing. One and half hours after the 35S label was removed, lysates were collected at increasing time points from 0 to 16 h. Samples were immunoprecipitated with anti-ERBB3 antibody and resolved by SDS-PAGE, and radiolabeled ERBB3 was visualized by autoradiography. For control samples (c), an ERBB3-derived blocking peptide was added during immunoprecipitation to determine the amount of nonspecific binding. c Geldanamycin and cycloheximide have comparable effects on the steady-state levels of ERBB3. MCF7 cells were treated with cycloheximide (CHX), GA, or CHX and GA together for 0–16 h. Cells were harvested; samples were resolved by SDS-PAGE and immunoblotted against ERBB2 or ERBB3 antibodies