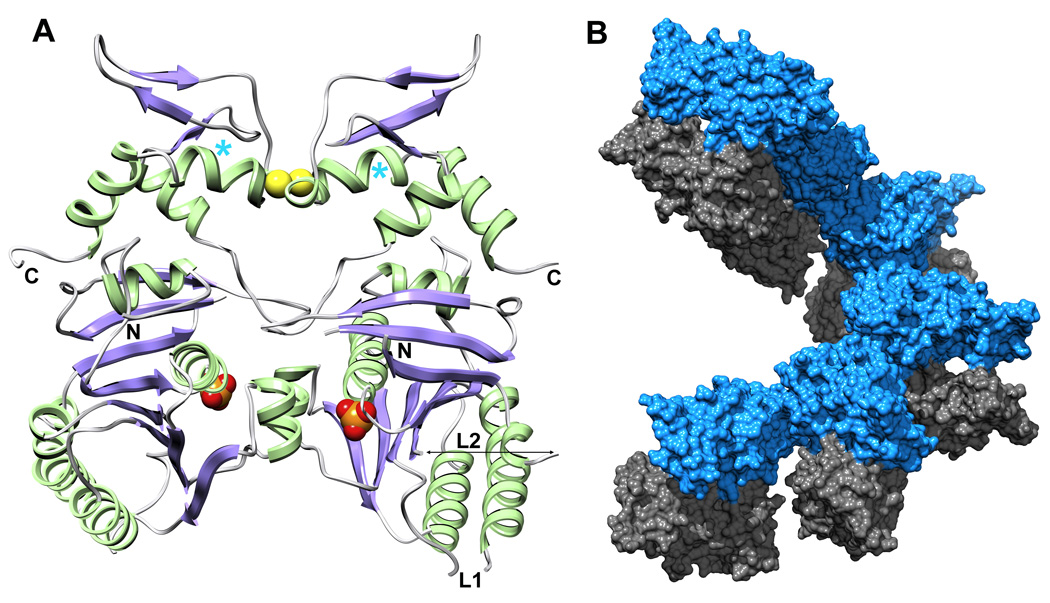
Fig. 2.
The dimeric arrangement of UvsX30–358 in the crystal asymmetric unit. (A) UvsX30–358 is colored as in Figure 1A. The ATP-binding sites are indicated by the phosphate groups (orange/red CPK), and are occluded at the dimer interface. The paired Cys316 residues at the dimer interface appear to form a partially occupied disulfide bridge (yellow CPK) in the crystal and in solution (see Figure 3). The positions of the N- and C-termini and Loops L1 and L2 are indicated. The positions of Pro322 highlighted in Figure 1C are marked with cyan asterisks. (B) Six dimers create one turn of a helical array in the P61 unit cell. Interactions between successive monomer A molecules (blue) mediate the helical filament with no contributions from monomer B (grey).