Abstract
AIM: To evaluate whether alcohol dehydrogenase-1B (ADH1B) His47Arg and aldehyde dehydrogenase-2 (ALDH2) Glu487Lys polymorphism is involved in the esophageal squamous cell carcinoma (ESCC) risk in Chinese Han population.
METHODS: Seven studies of ADH1B and ALDH2 genotypes in Chinese Han population in 1450 cases and 2459 controls were included for meta-analysis. Stratified analyses were carried out to determine the gene-alcohol and gene-gene interaction with ESCC risk. Potential sources of heterogeneity between studies were explored, and publication bias was also evaluated.
RESULTS: Individuals with ADH1B arginine (Arg)/Arg genotype showed 3.95-fold increased ESCC risk in the recessive genetic model [Arg/Arg vs Arg/histidine (His) + His/His: odds ratio (OR) = 3.95, 95% confidence interval (CI): 2.76-5.67]. Significant association was found in the dominant model for ALDH2 lysine (Lys) allele [glutamate (Glu)/Lys + Lys/Lys vs Glu/Glu: OR = 2.00, 95% CI: 1.54-2.61]. Compared with the non-alcoholics, Arg/Arg (OR = 25.20, 95% CI: 10.87-53.44) and Glu/Lys + Lys/Lys (OR = 21.47, 95% CI: 6.44-71.59) were found to interact with alcohol drinking to increase the ESCC risk. ADH1B Arg+ and ALDH2 Lys+ had a higher risk for ESCC (OR = 7.09, 95% CI: 2.16-23.33).
CONCLUSION: The genetic variations of ADH1B His47Arg and ALDH2 Glu487Lys are susceptible loci for ESCC in Chinese Han population and interact substantially with alcohol consumption. The individuals carrying both risky genotypes have a higher baseline risk of ESCC.
Keywords: Esophageal cancer, Alcohol metabolizing enzyme genes, Polymorphism, Susceptibility
INTRODUCTION
Esophageal carcinoma (EC) ranked as the eighth most common malignancy and the seventh leading cause of cancer death worldwide characterized by remarkable diversity according to geographical distribution. It is known that Asian countries, in particular China, Iran and Japan, have the highest incidence rates of EC in the world, and the esophageal squamous cell carcinoma (ESCC) is a predominant histological type. For example, in China, the age-standardized incidence rates of ESCC in males and females were 72-150/100 000 and 26-64/100 000, respectively, from 1995 to 2004 in a southern population[1]. ESCC is a complex disease influenced by environmental as well as genetic factors[2]. Behavioral factors include alcohol drinking which is common to all “high-risk populations”[3]. Odds ratio for ESCC was 50.1 for those who were both heavy drinkers and smokers in comparison with those who neither drank nor smoked[4]. Alcohol is not a carcinogen, and acetaldehyde is the most toxic alcohol metabolite in alcohol-associated carcinogenesis[2].
Efficiency in the conversion of ethanol to acetaldehyde and subsequent oxidation to acetate depends mainly on the alcohol dehydrogenase-1B (ADH1B) and the aldehyde dehydrogenase-2 (ALDH2) activities. Upon consumption of an alcoholic beverage, ethanol is first catalytically oxidized into acetaldehyde, occurring mainly through ADH1B. It is subsequently metabolized into harmless acetate, chiefly by ALDH2. Thus, genetic variants that result in functional differences in enzyme activity, lead to differences in acetaldehyde exposure among drinkers. A polymorphism in the ADH1B gene, resulting in an amino acid transition from arginine (Arg) to histidine (His) at codon 47 (Arg47His) in exon 3, bestows the super-active “fast” metabolic character on ethanol. About a 40-times greater maximum velocity has been identified for the ADH1B fast His allele than that for the less active Arg/Arg form[5,6]. In contrast, ALDH2 has a polymorphism, which results from the substitution of glutamate (Glu) to lysine (Lys) at residue 487 (also recognized as Glu504Lys) encodes a catalytically inactive subunit of ALDH2, whose ALDH2 Glu/Lys genotype has only 6.25% of the normal ALDH2 Glu protein[7].
In China, the ADH1B 47His allele frequency decreases dramatically from 90% to 10% from East China to West China, and its geographic distribution is consistent with the unearthed culture relic sites of rice domestication in China[8,9]. The ALDH2 487Lys allele is essentially absent in all parts of the world except East Asia and has the highest frequency in China, and is high in south coast and east coast of China, and decreased gradually toward inland China, west, northwest and north China. The origin of ALDH2 487Lys could be traced back to ancient Pai-Yuei tribe in south China[10]. Combined with the demographic history, the ADH1B 47His and ALDH2 487Lys are carried by Han Chinese as they spread throughout East Asia[11]. One effect of the ALDH2 487Lys mutation is the “Asian flush” - the red face, nausea, and rapid heartbeat that many people with East Asian ancestry experience when they drink alcohol. The alcohol flushing response (Asian Glow) is a biomarker for ALDH2 487Lys allele[12].
Various studies have focused on ADH1B and ALDH2 polymorphisms and the risk of ESCC in Chinese Han population[13-19]. These studies also suggested a gene-gene and gene-alcohol interaction for ESCC risk[18]. For example, the risk for ADH1B Arg/Arg carriers was significantly increased from 1.2 to 74 times in non-drinkers and drinkers, respectively, compared with non-drinkers who carried the ADH1B His/His genotype[15]. A meta-analysis of seven Asian (Chinese, Japanese, and Thailand) studies found that 487Lys allele was risky for ESCC susceptibility[20]. However, there was only one Chinese population for the meta-analysis. Meta-analysis can provide an opportunity to help identify genetic associations by overcoming the coalescent issues[21]. We performed a meta-analysis among all eligible studies to clarify the effect of ADH1B Arg47His and ALDH2 Glu487Lys polymorphism alone and interactively on ESCC risk in Chinese Han population.
MATERIALS AND METHODS
Selection criteria and identification of eligible studies
Eligible studies were identified by searching the database of PubMed for relevant articles in English. The latest searches were undertaken in January 2010. The following search terms: “esophageal cancer” or “esophageal cancer” and “ADH2” or “aldehyde dehydrogenase” or “ADH1B” or “ALDH2” “aldehyde dehydrogenase 2” “polymorphism” were used in isolation and combination with one another. Searches were limited to the studies involving human subjects and Chinese population. All population-based case-control studies focusing on the associations between the ADH1B Arg47His, ALDH2 Glu487Lys variants and ESCC risk were eligible for inclusion. Review articles, case reports, esophageal adenocarcinoma and repeated literatures were excluded. When overlapping articles were found, we only included the publication that reported the most extensive information.
The full-text of the candidate articles were examined carefully to determine whether they accorded with the inclusion criteria for the meta-analysis. A total of seven published studies with full-text articles examined the association of polymorphisms ADH1B Arg48His and ALDH2 Glu487Lys, and 3 of the seven were about the interaction between ADH1B and ALDH2 gene.
Data extraction
Data were extracted independently by Zhang GH and Mai RQ. We extracted the following information from each manuscript: author, year of publication, region, selection and characteristics of cancer cases and controls, and genotyping information.
Meta-analysis
The risk of ESCC associated with the ADH1B Arg47His and ALDH2 Glu487Lys variants were estimated for each study by odds ratio (OR) with 95% confidence intervals (CI) using comprehensive meta-analysis software version 2.0. For all studies, we evaluated the risk of the variant genotypes Arg/Arg or Arg/His, Lys/Lys or Glu/Lys and compared with the His/His and Glu/Glu genotypes. We then calculated the OR of the polymorphisms with dominant and recessive model. Between-study heterogeneity was estimated using the Cochran’s Q and χ2. Heterogeneity was considered statistically significant when P < 0.05. Point estimates and 95% CI were computed with both random effect and fixed effect models. If heterogeneity existed, point estimates and 95% CI were estimated on the basis of random effect model, Otherwise the fixed effect model was used. For the analysis of gene-gene interaction between ADH1B Arg47His and ALDH2 Glu487Lys, the His/His and Glu/Glu was considered as the reference genotype. For the gene-environment interaction, non-drinkers carrying the His/His genotype were set as compared subjects. Publication bias was assessed using funnel plots and Egger’s test. P < 0.05 was considered statistically significant. χ2 test was performed to examine the Hardy-Weinberg equilibrium (HWE) when genotype data were available.
RESULTS
Eligible studies and meta-analysis databases
Table 1 presents the characteristics of all the studies that were included in the meta-analyses. Seven studies published until July 2010 were about ADH1B Arg47His, ALDH2 Glu/Lys polymorphism and risk of ESCC, with a total number of 1450 cases and 2459 controls (Table 1). The genotype distribution in the control groups in each study did not depart from the HWE with P > 0.05.
Table 1.
Characteristics of studies included in meta-analysis
| Authors | Origin | HWE of controls | n | Controls |
| Ding et al[13], 2009 | Jiangsu | Yes | 191 | 221 |
| Guo et al[14], 2008 | Gansu | Yes | 80 | 480 |
| Lee et al[15], 2008 | Taiwan | Yes | 406 | 656 |
| Yang et al[16], 2007 | Sichuan | Yes | 191 | 198 |
| Chen et al[17], 2006 | Taiwan | Yes | 330 | 592 |
| Wu et al[18], 2005 | Taiwan | Yes | 134 | 237 |
| Chao et al[19], 2000 | Taiwan | Yes | 88 | 105 |
HWE: Hardy-Weinberg equilibrium.
Meta-analysis results
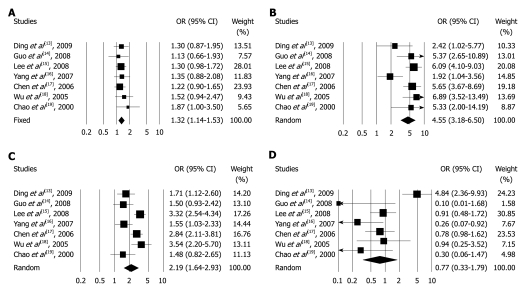
ADH1B Arg47His polymorphism: When the Arg/His and homozygote Arg/Arg were compared with the homozygous His/His genotype, the pooled ORs for all the 7 studies were 1.32 (95% CI: 1.14-1.53, P < 0.001, Phetero = 0.905) in the fixed model and 4.55 (95% CI: 3.18-6.50, P < 0.001, Phetero = 0.029) in the random model, respectively (Figure 1A and B). Similarly, increased associations were found in the dominant model, Arg/Arg + Arg/His vs His/His genotype, pooled OR was 1.62 (95% CI: 1.41-1.85, P < 0.001, Phetero = 0.206). Arg/Arg vs Arg/His + His/His genotype, pooled OR was 3.95 (95% CI: 2.76-5.67, P < 0.001, Phetero = 0.014) in the recessive model.
Figure 1.
Forest plot shows the odds ratios and confidence intervals of the association. A: Between arginine (Arg)/histidine (His) and His/His genotype of alcohol dehydrogenase-1B (ADH1B) His47Arg polymorphism; B: Between Arg/Arg and His/His genotype of ADH1B gene His47Arg polymorphism; C: Between glutamate (Glu)/lysine (Lys) and Gly/Glu genotype of aldehyde dehydrogenase-2 (ALDH2) Glu487Lys polymorphism; D: Between Lys/Lys and Glu/Glu genotype of ALDH2 Glu487Lys polymorphism. OR: Odds ratio; CI: Confidence interval.
ALDH2 Glu487lys polymorphism: The variant heterozygous genotype Glu/Lys carriers have a significant increased risk of ESCC compared with those carrying Glu/Glu genotype (OR = 2.19, 95% CI: 1.64-2.93, P < 0.001, Phetero < 0.001). But the homozygous genotype Lys/Lys did not show a significantly increased risk of ESCC (OR = 0.77, 95% CI: 0.33-1.79, P = 0.537, Phetero < 0.001) (Figure 1C and D). Similarly, no associations were found in the recessive model (Lys/Lys vs Glu/Lys + Glu/Glu: OR = 0.48, 95% CI 0.19-1.20, P = 0.118, Phetero < 0.001). Combining the homozygous Lys/Lys and heterozygous Glu/Lys genotypes, the pooled OR for the dominant model was 2.00 (Lys/Lys + Glu/Lys vs Glu/Glu: 95% CI: 1.54-2.61, P < 0.001, Phetero = 0.001).
Gene-environmental interactions: The joint associations of the ADH1B, ALDH2 polymorphism and alcohol drinking with the risk of ESCC were observed when the analysis was stratified by alcohol drinking status. Among non-drinkers, there was no evidence for an increased risk for ADH1B Arg/His vs His/His genotype of individuals (OR = 1.06, 95% CI: 0.75-1.50). However, compared with non-drinkers carrying ADH1B His/His genotype, drinkers carrying Arg/Arg and Arg/His genotype (OR = 6.97, 95% CI: 1.70-28.56) showed a significantly higher risk of ESCC, and the risk of homozygote Arg/Arg was highest (OR = 20.69, 95% CI: 5.09-84.13) among the subgroups of the three studies[13,15,17]. In the recessive model, when the drinking factor was integrated, statistically significantly elevated risks were found (Arg/Arg vs Arg/His + His/His: OR = 25.20, 95% CI: 10.87-53.44) (Table 2).
Table 2.
Interaction between alcohol drinking and genotype for esophageal squamous cell carcinoma risk
|
Non-drinker |
Drinker |
|||||
| Case/control | OR | 95% CI | Case/control | OR | 95% CI | |
| ADH1B[13-15,17,18] | ||||||
| His/His + Arg/His | 217/1355 | Ref. | - | 674/668 | 6.21 | 2.39-16.27 |
| Arg/Arg | 22/72 | 2.37 | 1.40-4.01 | 258/61 | 25.20 | 10.87-53.44 |
| ALDH2[13-18] | ||||||
| Glu/Glu | 96/604 | Ref. | - | 356/609 | 4.22 | 2.030-8.77 |
| Glu/Lys + Lys/Lys | 223/951 | 1.70 | 1.05-2.75 | 687/190 | 21.47 | 6.44-71.59 |
ADH1B: Alcohol dehydrogenase-1B; ALDH2: Aldehyde dehydrogenase-2; His: Histidine; Arg: Arginine; Glu: Glutamate; Lys: Lysine; OR: Odds ratio; CI: Confidence interval.
Odds ratios for the ALDH2 genotypes and the risk of ESCC were stratified by the alcohol consumption. Risk of ESCC was particularly high for drinkers with ALDH2 Glu/Lys genotype compared with non-drinkers having ALDH2 Glu/Glu genotype (OR = 23.83, 95% CI 3.75-151.05, P < 0.001) in the subgroups of five studies[13-15,17,18]. Furthermore, the homozygous Lys/Lys was found to interact with alcohol drinking to increase the ESCC risk (OR = 16.33, 95% CI: 5.21-51.17). In the dominant model, the OR risk was increased from 2.00 of null model to 21.47 of the interaction model.
Gene-gene interactions: Table 3 shows the interaction of ADH1B and ALDH2 genotypes on ESCC risk. Compared with subjects having ADH1B His/His with ALDH2 Glu/Glu, OR for those with ALDH2 Glu/Glu and ADH1B Arg+, ALDH2 Lys+ and ADH1B His/His, and ALDH2 Lys+ and ADH1B Arg+ was 3.66-13.46. The significantly increased risk for ESCC (OR = 7.09, 95% CI: 2.16-23.33, P < 0.001) was noted in individuals with ADH1B Arg+ and ALDH2 Lys+. Individuals carrying two risky alleles had an additional risk compared to those with only one risky allele.
Table 3.
Gene-gene interaction with alcohol dehydrogenase-1B and aldehyde dehydrogenase-2 for esophageal squamous cell carcinoma risk
Publication bias
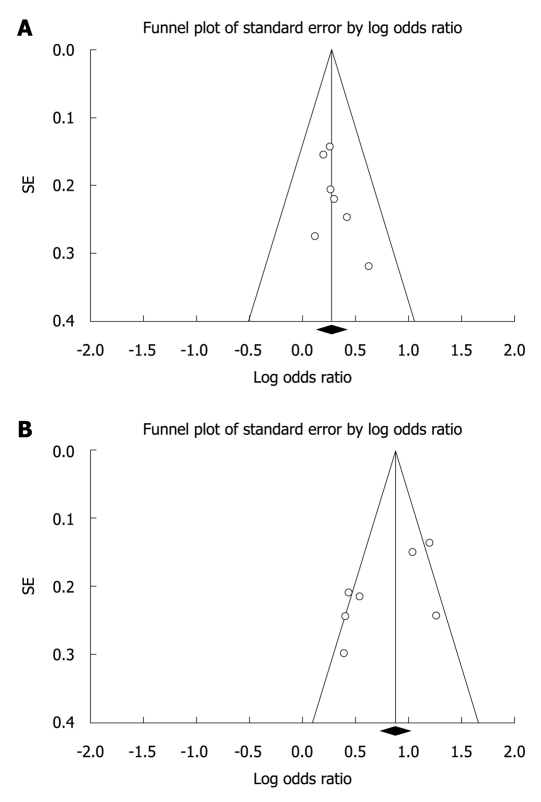
Funnel plot and Egger’s test were performed to assess the publication bias. No influence of publication bias was found in our study using Egger’s test P = 0.202 for the ADH1B, Egger’s test P = 0.085 for the ALDH2, as indicated by the funnel plot (Figure 2).
Figure 2.
Funnel plot of alcohol dehydrogenase-1B His47Arg (A) and aldehyde dehydrogenase-2 Glu487Lys (B) polymorphisms and esophageal squamous cell carcinoma risk for publication bias.
DISCUSSION
Several studies have demonstrated that the ADH1B and ALDH2 genotypes would be expected to result in exposure to high acetaldehyde concentrations[22]. A recent genome-wide association study showed that ADH1B Arg47His and ALDH2 Glu487Lys are associated with ESCC in a Japanese population[23]. The proportion of individuals carrying “susceptible genotypes of alcohol-related diseases” in Chinese Han healthy population was 68.16%[24]. In this meta-analysis, the pooled OR of ADH1B Arg47His polymorphism was 1.32 (95% CI: 1.14-1.53, P < 0.001, Phetero = 0.905) and 4.55 (95% CI: 3.18-6.50, P < 0.001, Phetero = 0.029) for Arg/His and homozygote Arg/Arg genotype in Chinese Han population, respectively. We also observed that ALDH2 Glu/Lys had independent and statistically significant effects on ESCC. Although there was no statistically significant increase of ESCC risk in the recessive model (OR = 0.48, 95% CI: 0.19-1.20, P = 0.118, Phetero < 0.001) and Lys/Lys genotype (OR = 0.77, 95% CI: 0.33-1.79, P = 0.537, Phetero < 0.001), the highest pooled OR was found in the Glu/Lys genotype (OR = 2.19, 95% CI: 1.64-2.93, P < 0.001, Phetero < 0.001). Our meta-analysis confirmed a positive association between the polymorphisms of ADH1B, ALDH2 and susceptibility to ESCC. The study by Zhou et al[25] found that Glu/Lys genotype was a risk factor for esophageal squamous cell dysplasia. Combining with our results, ALDH2 may be a candidate biomarker to screen early ESCC.
ADH1B and ALDH2 polymorphism was considered as risk-conferring factor for alcohol dependence[26], and many studies have indicated that the synergistic interaction between ADH1B and ALDH2 polymorphism and alcohol drinking are involved in the risk of ESCC[14,17,18]. Taking alcohol consumption and genetic vulnerability into consideration, this meta-analysis further identified an alcohol-genotype-dependent increase in ESCC risk for alcohol consumers. In this meta-analysis, the risk of individuals with ADH1B Arg/His genotype and alcohol drinking increased ESCC risk by 6.97 times (95% CI: 1.70-28.56). However, among non-drinkers, there is no strong evidence of an increased risk (OR = 1.06, 95% CI: 0.75-1.50). The magnitude of effect of ALDH2 Lys/Lys genotype was significant in alcohol drinkers (OR = 16.33, 95% CI: 5.21-51.17). Alcohol drinkers carrying ALDH2 Glu/Lys (OR = 23.83, 95% CI: 3.75-151.05) or ADH1B Arg/Arg (OR = 20.69, 95% CI: 5.09-84.13) had a higher risk of ESCC. These results might suggest that both the ADH1B Arg and ALDH2 Lys allele indicate a longer exposure to alcohol and highly-concentrated acetaldehyde, thus greatly increasing the susceptibility to ESCC. Alcohol has been intensified by the genetic modulation of ADH1B and ALDH2, which should make it more powerful. Furthermore, alcohol drinking with a combined genotype of ADH1B Arg/Arg and ALDH2 Glu/Lys was associated with increased DNA damage[27]. Avoidance of alcohol may be an important way to prevent ESCC among ADH1B Arg and ALDH2 Lys allele carriers.
There are many studies to explore the interaction between the ADH1B Arg/His and ALDH2 Glu487Lys polymorphism[14,15,18]. In this meta-analysis, we further found there was a highly significant gene-gene interactive effect between the two polymorphisms and ESCC risk. The risk effect of the interaction between ADH1B and ALDH2 was strongest in Arg+ and Lys+ carriers (OR: 7.09, 95% CI: 2.16-23.33), followed by Glu/Glu and Arg+ (OR = 3.66, 95% CI: 1.66-6.74) and Lys+ and His/His (OR = 2.72, 95% CI: 1.34-5.51) carriers. The results indicate that combined roles of the two genes and alcohol consumption should be considered to define the individual with ESCC risk.
We observed that the Arg/Arg and Glu/Lys genotypes were associated with increased risk for ESCC, but pooled OR was higher in carriers of the Arg/Arg (4.55) than in Glu/Lys carriers (2.00). Furthermore, the interactive risk of Glu/Glu and Arg+ (OR, 3.66) was higher than Lys+ and His/His (OR, 2.72). These results might suggest that the ADH1B has a predominant role in determining the risk of ESCC when the general population is considered.
Limitations
Although our primary result of this meta-analysis is suggestive, some limitations still exist. Firstly, we still lack more genotype data from the multiple highest incidence area, such as the Taihang Mountain in central China, Xinjiang and Chaoshan populations. Secondly, the number of studies included in this article was insufficient and the sample size of individual studies was also small. It is undoubted that larger studies should be done for confirming these findings, because our results are based on a limited sample size. Lastly, OR value was obtained without correction. More accurate OR should be corrected by age, gender and other environmental factors. A more precise analysis should be conducted if more detailed individual data are available, which would allow for an adjusted estimate. Adjusted covariates might help explain the association between ADH1B Arg47His and ALDH2 Glu/Lys polymorphism and susceptibility to ESCC.
In conclusion, this meta-analysis of seven case-control studies provided evidence that ADH1B Arg/His and ALDH2 Glu/Lys polymorphism was significantly associated with increased risk of ESCC. Gene-gene and gene-environment interactions are warranted to confirm the real contribution of these polymorphisms to ESCC susceptibility. Further studies in different high incidence areas of ESCC in China are also necessary to clarify the correlation between ADH1B Arg47His and ALDH2 Glu487Lys polymorphisms and ESCC risk.
COMMENTS
Background
Esophageal squamous cell carcinoma (ESCC) is the fourth most frequent cause of cancer-related deaths in China. Alcohol intake is positively associated with the risk of ESCC. The activity of alcohol dehydrogenase 1B (ADH1B) and acetaldehyde dehydrogenase 2 (ALDH2) is primarily responsible for the oxidative detoxification, and polymorphism His47Arg in ADH1B and Glu487Lys in ALDH2 modulate the conversion rate of acetaldehyde. These polymorphisms are most prevalent in East Asia and they have highest frequencies in Chinese Han population.
Research frontiers
Many case-control studies have been performed to evaluate the ADH1B and ALDH2 polymorphisms for esophageal cancer risk in Chinese Han population, including the role of gene-gene and gene-environment interaction in esophageal cancer risk. However, the evidence is insufficient with the small sample size.
Innovations and breakthroughs
This is the first meta-analysis which systemically studied the association between ADH1B and ALDH2 polymorphisms and esophageal cancer susceptibility in Chinese Han population, and suggested that ADH1B His47Arg and ALDH2 Glu487Lys are susceptible loci for ESCC and interact substantially with alcohol consumption. The individuals carrying the both genotypes have a higher baseline risk of ESCC.
Applications
This study provided a potential biomarker to identify high-risk individuals for esophageal cancer in Chinese Han population, specially among the alcohol drinkers.
Terminology
ADH1B: A member of the alcohol dehydrogenase family and the major enzyme that catalyzes alcohol to acetaldehyde in liver. ALDH2: A member of aldehyde dehydrogenases, and is the second enzyme of the major oxidative pathway of alcohol metabolism.
Peer review
The data and their interpretations are reasonable. This paper has a certain value in this research field because previous studies had a low statistical power because of the small sample size.
Footnotes
Supported by The National Natural Science Foundation of China, No. 30901726
Peer reviewer: Satoshi Osawa, MD, First Department of Medicine, Hamamatsu University School of Medicine, 1-20-1 Handayama, Hamamatsu, 431-3192, Japan
S- Editor Sun H L- Editor Ma JY E- Editor Lin YP
References
- 1.Su M, Liu M, Tian DP, Li XY, Zhang GH, Yang HL, Fan X, Huang HH, Gao YX. Temporal trends of esophageal cancer during 1995-2004 in Nanao Island, an extremely high-risk area in China. Eur J Epidemiol. 2007;22:43–48. doi: 10.1007/s10654-006-9086-x. [DOI] [PubMed] [Google Scholar]
- 2.Toh Y, Oki E, Ohgaki K, Sakamoto Y, Ito S, Egashira A, Saeki H, Kakeji Y, Morita M, Sakaguchi Y, et al. Alcohol drinking, cigarette smoking, and the development of squamous cell carcinoma of the esophagus: molecular mechanisms of carcinogenesis. Int J Clin Oncol. 2010;15:135–144. doi: 10.1007/s10147-010-0057-6. [DOI] [PubMed] [Google Scholar]
- 3.Zheng S, Vuitton L, Sheyhidin I, Vuitton DA, Zhang Y, Lu X. Northwestern China: a place to learn more on oesophageal cancer. Part one: behavioural and environmental risk factors. Eur J Gastroenterol Hepatol. 2010;22:917–925. doi: 10.1097/MEG.0b013e3283313d8b. [DOI] [PubMed] [Google Scholar]
- 4.Morita M, Kumashiro R, Kubo N, Nakashima Y, Yoshida R, Yoshinaga K, Saeki H, Emi Y, Kakeji Y, Sakaguchi Y, et al. Alcohol drinking, cigarette smoking, and the development of squamous cell carcinoma of the esophagus: epidemiology, clinical findings, and prevention. Int J Clin Oncol. 2010;15:126–134. doi: 10.1007/s10147-010-0056-7. [DOI] [PubMed] [Google Scholar]
- 5.Smith M. Genetics of human alcohol and aldehyde dehydrogenases. Adv Hum Genet. 1986;15:249–290. doi: 10.1007/978-1-4615-8356-1_5. [DOI] [PubMed] [Google Scholar]
- 6.Bosron WF, Li TK. Genetic polymorphism of human liver alcohol and aldehyde dehydrogenases, and their relationship to alcohol metabolism and alcoholism. Hepatology. 1986;6:502–510. doi: 10.1002/hep.1840060330. [DOI] [PubMed] [Google Scholar]
- 7.Crabb DW, Edenberg HJ, Bosron WF, Li TK. Genotypes for aldehyde dehydrogenase deficiency and alcohol sensitivity. The inactive ALDH2(2) allele is dominant. J Clin Invest. 1989;83:314–316. doi: 10.1172/JCI113875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peng Y, Shi H, Qi XB, Xiao CJ, Zhong H, Ma RL, Su B. The ADH1B Arg47His polymorphism in east Asian populations and expansion of rice domestication in history. BMC Evol Biol. 2010;10:15. doi: 10.1186/1471-2148-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borinskaya S, Kal'ina N, Marusin A, Faskhutdinova G, Morozova I, Kutuev I, Koshechkin V, Khusnutdinova E, Stepanov V, Puzyrev V, et al. Distribution of the alcohol dehydrogenase ADH1B*47His allele in Eurasia. Am J Hum Genet. 2009;84:89–92; author reply 92-94. doi: 10.1016/j.ajhg.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luo HR, Wu GS, Pakstis AJ, Tong L, Oota H, Kidd KK, Zhang YP. Origin and dispersal of atypical aldehyde dehydrogenase ALDH2487Lys. Gene. 2009;435:96–103. doi: 10.1016/j.gene.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 11.Li H, Borinskaya S, Yoshimura K, Kal'ina N, Marusin A, Stepanov VA, Qin Z, Khaliq S, Lee MY, Yang Y, et al. Refined geographic distribution of the oriental ALDH2*504Lys (nee 487Lys) variant. Ann Hum Genet. 2009;73:335–345. doi: 10.1111/j.1469-1809.2009.00517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brooks PJ, Enoch MA, Goldman D, Li TK, Yokoyama A. The alcohol flushing response: an unrecognized risk factor for esophageal cancer from alcohol consumption. PLoS Med. 2009;6:e50. doi: 10.1371/journal.pmed.1000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding JH, Li SP, Cao HX, Wu JZ, Gao CM, Su P, Liu YT, Zhou JN, Chang J, Yao GH. Polymorphisms of alcohol dehydrogenase-2 and aldehyde dehydrogenase-2 and esophageal cancer risk in Southeast Chinese males. World J Gastroenterol. 2009;15:2395–2400. doi: 10.3748/wjg.15.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo YM, Wang Q, Liu YZ, Chen HM, Qi Z, Guo QH. Genetic polymorphisms in cytochrome P4502E1, alcohol and aldehyde dehydrogenases and the risk of esophageal squamous cell carcinoma in Gansu Chinese males. World J Gastroenterol. 2008;14:1444–1449. doi: 10.3748/wjg.14.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee CH, Lee JM, Wu DC, Goan YG, Chou SH, Wu IC, Kao EL, Chan TF, Huang MC, Chen PS, et al. Carcinogenetic impact of ADH1B and ALDH2 genes on squamous cell carcinoma risk of the esophagus with regard to the consumption of alcohol, tobacco and betel quid. Int J Cancer. 2008;122:1347–1356. doi: 10.1002/ijc.23264. [DOI] [PubMed] [Google Scholar]
- 16.Yang SJ, Wang HY, Li XQ, Du HZ, Zheng CJ, Chen HG, Mu XY, Yang CX. Genetic polymorphisms of ADH2 and ALDH2 association with esophageal cancer risk in southwest China. World J Gastroenterol. 2007;13:5760–5764. doi: 10.3748/wjg.v13.i43.5760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen YJ, Chen C, Wu DC, Lee CH, Wu CI, Lee JM, Goan YG, Huang SP, Lin CC, Li TC, et al. Interactive effects of lifetime alcohol consumption and alcohol and aldehyde dehydrogenase polymorphisms on esophageal cancer risks. Int J Cancer. 2006;119:2827–2831. doi: 10.1002/ijc.22199. [DOI] [PubMed] [Google Scholar]
- 18.Wu CF, Wu DC, Hsu HK, Kao EL, Lee JM, Lin CC, Wu MT. Relationship between genetic polymorphisms of alcohol and aldehyde dehydrogenases and esophageal squamous cell carcinoma risk in males. World J Gastroenterol. 2005;11:5103–5108. doi: 10.3748/wjg.v11.i33.5103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chao YC, Wang LS, Hsieh TY, Chu CW, Chang FY, Chu HC. Chinese alcoholic patients with esophageal cancer are genetically different from alcoholics with acute pancreatitis and liver cirrhosis. Am J Gastroenterol. 2000;95:2958–2964. doi: 10.1111/j.1572-0241.2000.02328.x. [DOI] [PubMed] [Google Scholar]
- 20.Lewis SJ, Smith GD. Alcohol, ALDH2, and esophageal cancer: a meta-analysis which illustrates the potentials and limitations of a Mendelian randomization approach. Cancer Epidemiol Biomarkers Prev. 2005;14:1967–1971. doi: 10.1158/1055-9965.EPI-05-0196. [DOI] [PubMed] [Google Scholar]
- 21.Lohmueller KE, Pearce CL, Pike M, Lander ES, Hirschhorn JN. Meta-analysis of genetic association studies supports a contribution of common variants to susceptibility to common disease. Nat Genet. 2003;33:177–182. doi: 10.1038/ng1071. [DOI] [PubMed] [Google Scholar]
- 22.Yang CX, Matsuo K, Ito H, Hirose K, Wakai K, Saito T, Shinoda M, Hatooka S, Mizutani K, Tajima K. Esophageal cancer risk by ALDH2 and ADH2 polymorphisms and alcohol consumption: exploration of gene-environment and gene-gene interactions. Asian Pac J Cancer Prev. 2005;6:256–262. [PubMed] [Google Scholar]
- 23.Cui R, Kamatani Y, Takahashi A, Usami M, Hosono N, Kawaguchi T, Tsunoda T, Kamatani N, Kubo M, Nakamura Y, et al. Functional variants in ADH1B and ALDH2 coupled with alcohol and smoking synergistically enhance esophageal cancer risk. Gastroenterology. 2009;137:1768–1775. doi: 10.1053/j.gastro.2009.07.070. [DOI] [PubMed] [Google Scholar]
- 24.Cao XR, Wu DS. [Polymorphism of alcohol metabolizing-related enzyme genes and its correlation with drinking-behaviors in 201 cases of Chinese Han healthy population.] Zhonghua Yufang Yixue Zazhi. 2005;39:84–87. [PubMed] [Google Scholar]
- 25.Zhou YZ, Diao YT, Li H, Li HQ, Ma Q, Cui J. Association of genetic polymorphisms of aldehyde dehydrogenase-2 with esophageal squamous cell dysplasia. World J Gastroenterol. 2010;16:3445–3449. doi: 10.3748/wjg.v16.i27.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luczak SE, Glatt SJ, Wall TL. Meta-analyses of ALDH2 and ADH1B with alcohol dependence in Asians. Psychol Bull. 2006;132:607–621. doi: 10.1037/0033-2909.132.4.607. [DOI] [PubMed] [Google Scholar]
- 27.Weng H, Weng Z, Lu Y, Nakayama K, Morimoto K. Effects of alcohol-drinking behaviour and ADH1B and ALDH2 polymorphisms on basal DNA damage in human mononuclear cells as determined by the comet assay. Mutat Res. 2010;701:132–136. doi: 10.1016/j.mrgentox.2010.05.013. [DOI] [PubMed] [Google Scholar]