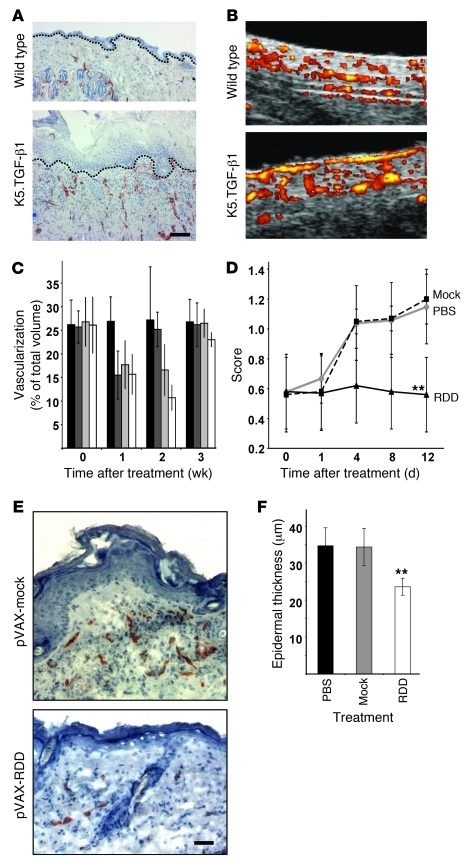
Figure 3. Therapeutic efficacy of RDD gene therapy in K5.TGF-β1 transgenic mice.
(A) Immunohistochemical analysis of CD31 in back skin of a wild-type and a K5.TGF-β1 transgenic littermate. Transgenic skin shows more and larger blood vessels than wild-type skin. Scale bar: 50 μm. Dashed lines, dermo-epidermal junction. (B) Blood flow within the upper cutaneous plexus was analyzed in vivo in a wild-type (top) and a K5.TGF-β1 transgenic mouse (bottom) by high-resolution Doppler ultrasound. Flowing blood as a surrogate marker for vascularization is visualized as yellow and red. Original magnification, ~×100. (C) K5.TGF-β1 transgenic mice (n = 4 in each group) were subjected to gene therapy with pVAX-mock (black bars) or pVAX-RDD at doses of 12.5 μg (dark gray bars), 25 μg (light gray bars), or 50 μg (white bars). The cutaneous blood flow in all mice was determined by high-resolution Doppler ultrasound. (D) Clinical severity scores in K5.TGF-β1 transgenic mice (n = 8 in each group) subjected to electroporation only (PBS, gray diamonds) or to gene therapy with pVAX-mock (filled squares) or pVAX-RDD (triangles) were monitored. Values represent mean ± SD. **P < 0.01. (E) Cryostat-cut skin sections of a K5.TGF-β1 transgenic mouse subjected to gene therapy using the pVAX-mock plasmid (top panel) and a littermate treated with pVAX-RDD (bottom panel) were analyzed by immunohistochemistry using a CD31-directed antibody 12 days after treatment. Scale bar: 20 μm. (F) Morphometric analysis of epidermal thickness in K5.TGF-β1 transgenic mice (n = 8 in each group) subjected to electroporation only (PBS, black bar) or to gene therapy with pVAX-mock (gray bar) or pVAX-RDD (white bar). **P < 0.01 compared with the pVAX-mock controls.