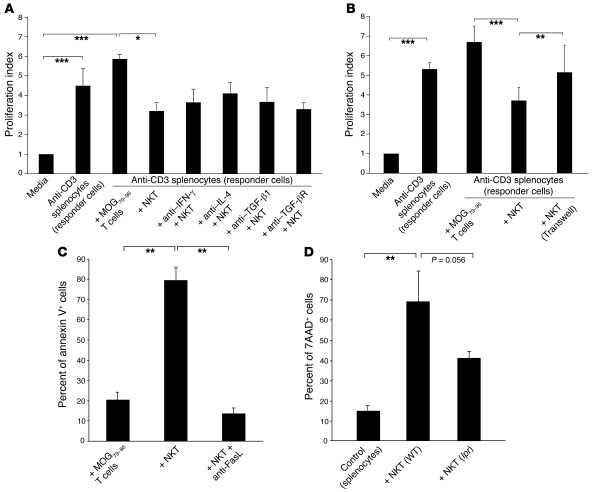
Figure 8. mCII707–721–specific NKT cells have a suppressive function.
(A and B) Suppressor assay was performed using in vitro–stimulated anti-CD3 splenocytes (responder cells) from B10.Q mice, cocultured for 64 hours with either mCII707–721–specific NKT cell line or B10.Q-restricted MOG79–96–specific T cell line (control) at a 1:1 ratio. Proliferation index was calculated by normalizing all cpm values to media control as 1. (A) Blocking IFN-γ, IL-4, TGF-β1, and TGF-β receptor with antibodies (20 μg/ml) had no effect on NKT cell suppression compared with isotype-matched controls; therefore suppression did not operate through cytokine release from mCII707–721–specific NKT cells. Data are mean ± SD, n = 3. *P ≤ 0.05; ***P ≤ 0.001. (B) Significantly reduced proliferation of anti-CD3–stimulated responder cells was observed when cultured with the mCII707–721–specific NKT cells (with or without Transwell system) compared with control cells. Proliferation of the responder cells was restored when a Transwell system inhibited cell-cell contact. Data are mean ± SD, n = 2–3. **P ≤ 0.01; ***P ≤ 0.001. (C) Cell death by annexin V+ FACS analysis of cocultured splenocytes (CFSE labeled), showing that the mCII707–721–specific NKT cell line induced significantly elevated cell death levels compared with the control cells. Anti-FasL (20 μg/ml) before coculturing revealed that splenocyte killing was mediated by FasL interaction. Data are mean ± SD, n = 3. **P ≤ 0.01. (D) Splenocytes (CFSE labeled) from WT mice stimulated with plate-bound anti-CD3 and cocultured for 48 hours with NKT cells purified from mCII707–721–immunized WT mice or lpr mice. Cell death was determined by 7AAD staining. Data are mean ± SD, n = 10 mice per group. **P ≤ 0.01.