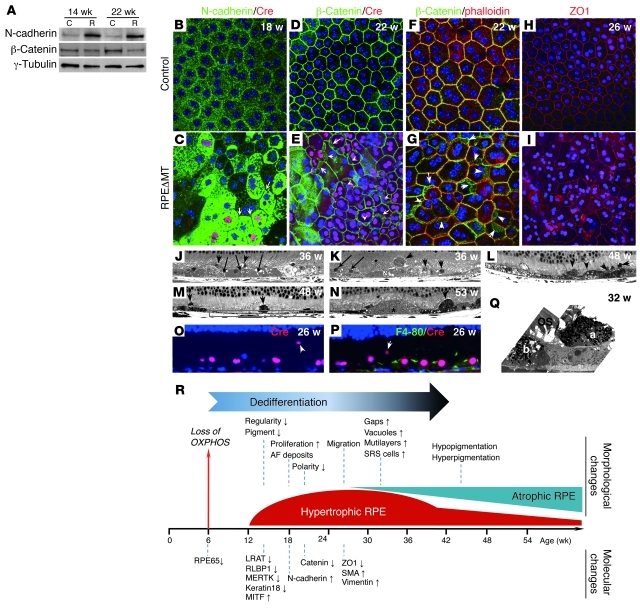
Figure 3. Decreased junctional integrity and increased migration of RPE cells in RPEΔMT mice.
(A) Immunoblot of RPE cells from pigmented RPEΔMT mice shows increased N-cadherin and reduced β-catenin. (B–I) Immunostaining of RPE flat mounts from RPEΔMT mice (C, E, G, and I) shows N-cadherin (green) substantially increased in RPE cytoplasm but diminished at cell junctions (C, arrows denote cell junctions). β-Catenin staining (green) is obscured at cre-expressing cell (red) boundaries, with a cytoplasmic redistribution (E, arrows), which is confirmed by co-labeling of β-catenin (green) and phalloidin (red) (G, arrows). (I) A stacked Z-series confocal image (15-μm thickness) shows diminished ZO1 staining (red) at RPE cell junctions. Original magnification, ×400. (J–N) Light microscopy of albino (J and K) and pigmented (L–N) RPEΔMT mice demonstrates gaps between RPE cells (J, arrows), RPE vacuoles (J–L), multilayered RPE cells (K, arrows), and numerous cells in the subretinal space (arrowheads in J–N), which leads to an uneven RPE appearance at late stages as well as RPE atrophy (boxed area in M). (N) Asterisks mark drusen-like material. (O and P) Cre-expressing RPE cells (red) are present in the subretinal space (arrowheads), whereas F4-80 reactivity (green), indicative of macrophages/microglia, is restricted to less than 2% of the RPE layer at 26 weeks. (J–P) Original magnification, ×630. (Q) An electron micrograph from an RPEΔMT mouse shows 2 pigmented cells of similar appearance (a and b), 1 of which a has migrated to the subretinal space. Original magnification, ×5000. (R) Loss of OXPHOS causes a sequence of morphological and molecular changes, beginning with a process of RPE dedifferentiation. Up and down arrows denote increased and decreased expression, repectively.