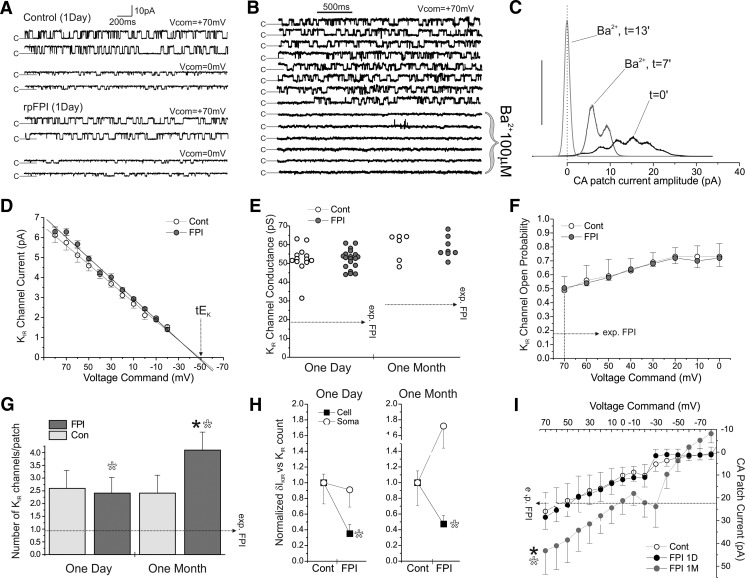
Fig. 2.
Neocortical astrocytic somata present no change in KIR current at 1 day and a compensatory increase at 1 mo after rpFPI. Cell-attached recordings from astrocytic somata do not show loss of IKIR, suggesting the IKIR loss observed in whole cell is from the impairment of astrocytes' processes. A: channel gating (c = closed) during cell-attached recordings from control and rpFPI neocortical astrocytic somata at Vcom of +70 and 0 mV. B: segments of consecutive sweeps obtained over 15 min from a cell-attached patch containing 1 active KIR channel before and during Ba2+ (100 μM) application through the pipette. Channel opening is potently blocked by Ba2+ as expected for KIR channels. C: all-points histograms of KIR channel activity during application of 100 μM Ba2+ through the pipette to a patch bearing multiple channels. Peaks at t = 0 indicate opening of multiple channels before Ba2+ application. Peaks are left-shifted and consolidated over time, indicating Ba2+ blockade of all channels on the patch. At t = 13 min, all channels are permanently closed. Y scale in arbitrary units. D: current-voltage plots for control and rpFPI channels recorded in somatic cell-attached patches show the same reversal potential (Vcom ≈ −48mV) consistent with a theoretical EK (tEK) in cell-attached of ≈ −22 mV, assuming cell Vm ≈ −70 mV, [K+]i = 66 mM, and [K+]pipette = 140 mM. E: slope conductance of cell-attached KIR channels from control and FPI rats 1 day and 1 mo after FPI. No injury-induced changes in channel conductance are observed. F: voltage dependence of Po in channels from control and 1 day FPI patches. No injury-induced changes in channel Po are observed. G: numbers of KIR channels found in cell-attached patches obtained 1 day and 1 mo after injury. No changes are observed 1 day after rpFPI, when the whole cell δIKIR deficit is most severe, whereas an increase in number of KIR channels is observed 1 mo after injury. Filled asterisk indicates statistical significant difference of rpFPI (1 mo) compared with a pooled control incorporating all other groups (P = 0.027). Hollow asterisks indicate statistical significance of the 2-way ANOVA test shown in H. H: summary of the 2-way ANOVA indicating a statistically significant difference between the effects of rpFPI on whole cell δIKIR (Cell) and on cell-attached KIR channel count (Soma). Cell values are normalized to the mean of the control groups. Asterisks indicate statistical significance: 1 day, P = 0.025; 1 mo, P = 0.002. I: I-V plot of mean cell-attached patch currents (corrected for leak current through the gigaseal) recorded during applied potentials from +70 to −70 mV in control and FPI at 1 day and 1 mo after injury. Currents measured 1 day after FPI are similar to control but are increased at 1 mo after FPI. Filled asterisk indicates P < 0.05 compared with controls. Empty asterisk indicates the significant difference (treatment × technique interaction) in the effect of rpFPI on somatic cell-attached vs. whole cell inward currents measured in the same cells (P = 0.003). Horizontal dotted arrows (exp. FPI) in E–I indicate the expected changes required to fully account for the whole cell KIR deficit after FPI.