Abstract
Visually guided hand movements in primates require an interconnected network of various cortical areas. Single unit firing rate from area 7a and dorsal prelunate (DP) neurons of macaque posterior parietal cortex (PPC) was recorded during reaching movements to targets at variable locations and under different eye position conditions. In the eye position–varied task, the reach target was always foveated; thus eye position varied with reach target location. In the retinal-varied task, the monkey reached to targets at variable retinotopic locations while eye position was kept constant in the center. Spatial tuning was examined with respect to temporal (task epoch) and contextual (task condition) aspects, and response fields were compared. The analysis showed distinct tuning types. The majority of neurons changed their gain field tuning and retinotopic tuning between different phases of the task. Between the onset of visual stimulation and the preparatory phase (before the go signal), about one half the neurons altered their firing rate significantly. Spatial response fields during preparation and initiation epochs were strongly influenced by the task condition (eye position varied vs. retinal varied), supporting a strong role of eye position during visually guided reaching. DP neurons, classically considered visual, showed reach related modulation similar to 7a neurons. This study shows that both area 7a and DP are modulated during reaching behavior in primates. The various tuning types in both areas suggest distinct populations recruiting different circuits during visually guided reaching.
INTRODUCTION
Primates rely heavily on the visual guidance of limb movements for foraging and social interactions. They have to select a visual target, move the eyes to the target, and finally follow with the hand to the target (Desmurget and Grafton 2000). This reaching process requires transformations between coordinate systems in time with multiple computational steps involved (Shadmehr and Wise 2005). Areas of the posterior parietal cortex (PPC) play a critical role in the transformation between vision and action by combining signals from various cortical areas.
Eye position modulates visual neural responsiveness of PPC neurons (Andersen and Mountcastle 1983; Andersen et al. 1985, 1990b; Read and Siegel 1997; Salinas and Sejnowski 2001). Reaching studies of the parietal reach region (PRR) suggest that visually guided arm movements are planned in eye-centered coordinates (Batista et al. 1999; Buneo et al. 2002; Scherberger et al. 2005; Snyder et al. 2006). In this study, eye position was either varied together with the reach target so that reaching was made to foveated targets, or fixation was kept constant on the center so that reaching was made to nonfoveated targets. The goal of this study was to explore the specific spatial and temporal relationships between visual, preparatory and reach signals in two regions of the PPC. Area 7a and the nearby dorsal prelunate (DP), which is at the most posterior end of the PPC and believed to be strongly visual, were examined. The DP region has strong feedforward connections to area 7a (Andersen et al. 1990a; Cavada and Goldman-Rakic 1989a), but also receives signals from other parietal, frontal, and extrastriate visual areas (Stepniewska et al. 2005).
The first hypothesis tested whether eye position and retinotopic tuning of 7a and DP neurons were affected by task phase, especially preparation and initiation of the reaching movement. If different mechanisms or inputs contributed to various signals in PPC such as eye position and motor planning, we might expect differential effects on spatial tuning for the visual, preparatory, and movement initiation phases of the task. The second hypothesis examined the spatial parameters during preparation and initiation of the reaching movement between the two task conditions, that is, whether the reach targets were foveated. Planning and executing a reach movements to targets in the same body-centered, but different eye-centered coordinates, might yield distinct spatial tunings of 7a and DP neurons because both areas are strongly altered by gain fields.
We implemented an “approach” (Gardner et al. 2007), also called “radial” (Fattori et al. 2005) reaching movement, in which the hand starts from a position close to the animal's trunk and moves in three dimensions (3D) toward the reach target, as used in an area 7a study by MacKay (1992). We used optic flow field stimuli as reach targets. This stimulus ensured that we activated 7a and DP neurons optimally and allowed to study the influence of the reach-related signals modulating these visual responses. The delayed reaching task evaluated visual stimulus presentation, eye position, preparation, and initiation of the arm movement activity.
METHODS
Animal preparation
Two male rhesus monkeys (M1R, 11 kg; M3R, 8.5 kg; age ∼10 yr) were trained on a visually guided reaching task. All procedures conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals; Rutgers University Animal Care and Facilities Committee approved all experimental protocols.
Each animal was prepared for electrophysiological recordings by attaching a head post and recording chamber in separate surgeries. All surgeries were performed under sterile conditions with isoflurane anesthesia. Both monkeys had been previously used for intrinsic optical imaging of posterior parietal cortex and had imaging chambers (diameter 20 mm) implanted over their right hemisphere (Heider et al. 2005; Siegel et al. 2003) contralateral to the left hand used for reaching. This permitted visual identification of areas 7a and DP in the chamber. At the conclusion of the optical imaging studies and before recording, the transparent artificial dura was removed, and the natural dura was allowed to grow back over the brain. If necessary, systemic prophylactic antibiotics were given for this procedure (Ceftriaxone, 50 mg/kg). A stainless steel adapter was attached to the optical chamber to hold the stage and microdrive and allow precise, targeted electrode penetrations into designated areas (Fig. 1).
Fig. 1.
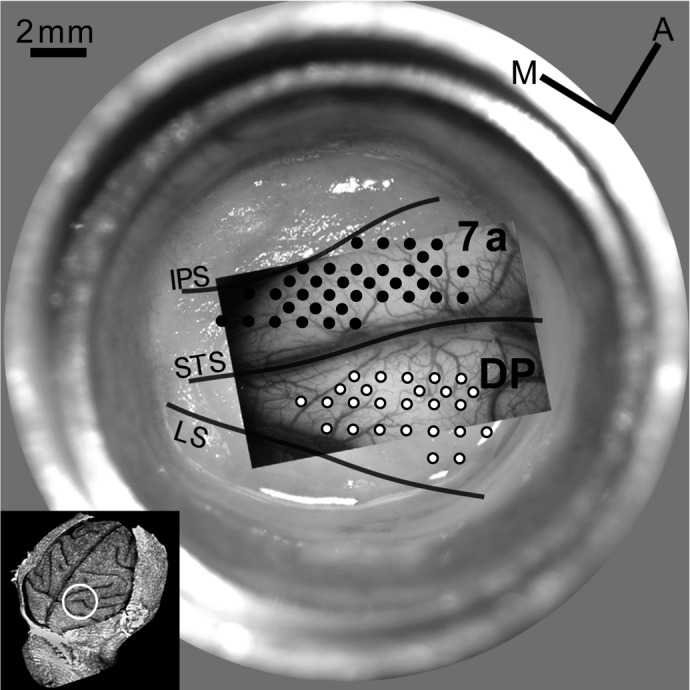
Recording sites in optical chamber (M1R). Image of blood vessel pattern (green light, 540 nm) and grid with recording sites overlaid on chamber picture with regrown dura. Area 7a is located between intraparietal sulcus (IPS) and superior temporal sulcus (STS; filled circles mark recording sites); the dorsal prelunate (DP) is located between lunate sulcus (LS) and STS (open circles mark recording sites). Grid, 1-mm spacing. Small inset shows anatomical overview of the right hemisphere of M1R reconstructed from structural MRIss. White circle marks location of recording chamber relative to the sulcal pattern.
Experimental setup
During the experiment, the animal's head was fixed with the head post attached to a specially designed primate chair that allowed free movement of the upper limbs. A touch-sensitive panel (Crist Instrument, Hagerstown, MD) or capacitive proximity sensor (IFM Efector, Exton, PA) attached to the belly plate of the primate chair ensured that the monkey held his reaching hand in a constant launching position close to his torso. A touch sensitive monitor (Elo TouchSystems, Menlo Park, CA) positioned 30 or 35 cm (depending on each monkey's arm length) from the animal's eyes recorded the reaching endpoints. Distance from the launching position to the touch screen was 35–40 cm (depending on the end position of the target). The reach distance from the start panel to the nine end positions was not varied in a systematic manner. The experiments were performed in a completely dark room. However, a faint luminosity of the touch screen could not be completely eliminated even at the lowest brightness settings and completely black background. Thus the monkey could only perceive his hand when it had reached and partially occluded the reach target.
The NIMH Cortex software (//www.cortex.salk.edu) displayed the stimuli, controlled the behavioral variables, and was synchronized to the analog spike collection system that was programmed using Matlab (MathWorks, Natick, MA) in conjunction with aPCMCIA-based A/D converter (NI DAQCard-6036E, National Instruments, Austin, TX) in a PC-based system.
Stimuli and behavioral task
The fixation dot consisted of a small (0.8° diam) red square. The visual reach targets were circular patches of moving dots (12° diam). These were either expansion (76%) or compression (24%) optic flow patches, which consisted of 128 dots (0.1° diam) with an equivalent angular velocity of 6°/s and a point life of 532 ms. The optic flow patches were displayed in one of nine positions within a 3 × 3 grid (step 12°, total 36 × 36° area).
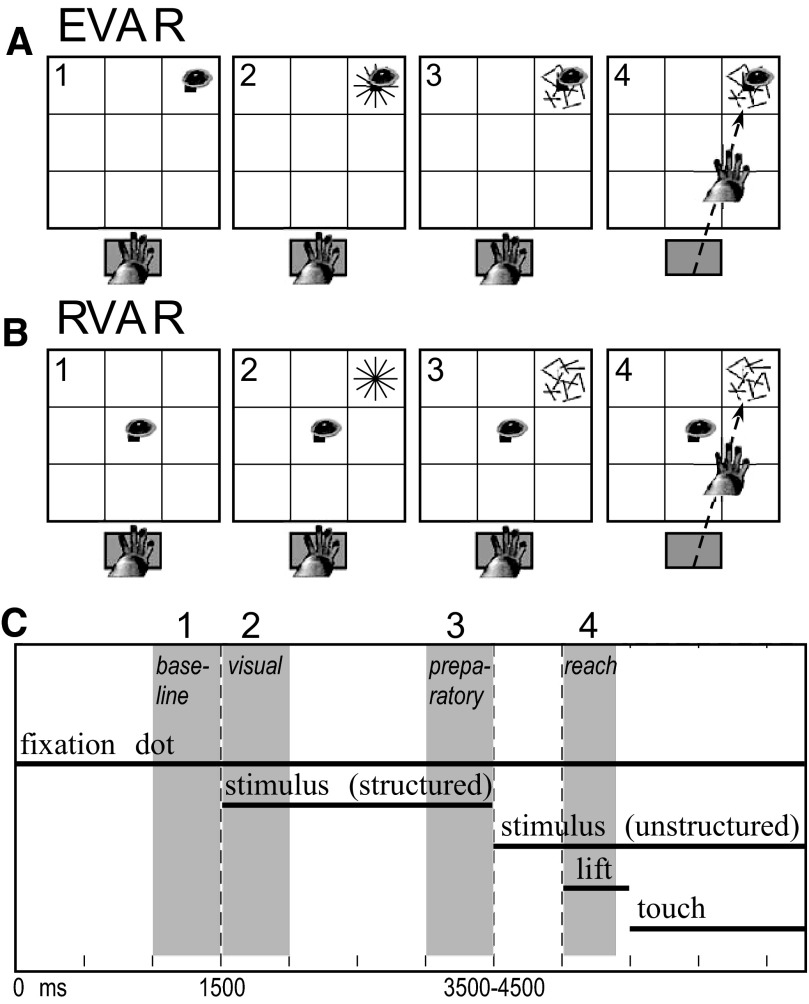
The monkeys performed two versions of the reaching task. In the eye position–varied task (EVAR; Fig. 2A), the small fixation dot and the optic flow reach target were presented together in one of the nine positions to test the effect of eye position. The optic flow patch was presented behind the fixation dot, which was visible throughout the task. Thus the reach target was always foveated ensuring that angle of gaze and reach position varied jointly, whereas the locus of retinotopic stimulation remained constant over the fovea. In the retinal-varied task (RVAR; Fig. 2B), the fixation dot always appeared in the center of the screen (primary position 0, 0°), and the optic flow target was displayed in one of the nine positions. Thus the reach target was not foveated, except for the center target, and retinotopic stimulation was varied. In both tasks, the monkeys were required to touch the targets within the 12°-target diameter.
Fig. 2.
Display for (A) the eye position–varied (EVAR) and (B) retinal-varied tasks (RVAR) and (C) task sequence. Numbers in each panel indicate epochs of interest. 1) Baseline fixation period with hand on the launch panel (1,500 ms). The fixation dot appears immediately after the animal places his hand on the launch panel. 2) Visual stimulation period (optic flow reach target) during which the hand remains on the launch panel (2,000–3,000 ms). The stimulus appears at the top right location (coordinates 12,12°) in the schematic (A and B). 3) Stimulus change (structured to unstructured motion of optic flow) instructs the monkey to lift his hand off the panel. 4) Reach period after the hand is lifted off the panel. Throughout the task, the monkey has to maintain fixation on the fixation dot. Epochs for analysis of neural activity are indicated by gray-shaded rectangles.
The temporal sequence of task events was always as follows (Fig. 2C). A trial started only when the left hand rested on the touch-sensitive launching panel. Then, the fixation dot appeared and the monkey started fixating within 500 ms. After 1,500 ms, the optic flow target was displayed. Randomized between 2,000 and 3,000 ms after stimulus onset, the structured optic flow changed to unstructured motion; thus the duration of the delay period was variable. Within a 600-ms reaction time, the animal made a hand movement to the target. Once the hand landed on the touch screen, the monkey had to hold his hand on the optic flow target for 1,500 ms. The target remained in place during this period. A drop of juice rewarded the animal for successful completion of the trial.
If the monkey did not acquire fixation within the 500-ms time, the trial was aborted, and a new trial started after 500 ms. Launching the hand incorrectly, that is, too early before or too late after the stimulus changed, terminated the trial immediately. The monkey performed a minimum of 10 trials per condition (total of 90 correct responses for either RVAR or EVAR task). The two tasks were performed in separate blocks of trials with their order varied.
An infrared eye camera (ISCAN, Cambridge, MA) in conjunction with the NIMH Cortex system monitored and collected eye position noninvasively at 60 Hz. The eye movement window was kept at 4° to control fixation; this value conforms to other reaching studies (Batista and Andersen 2001; Battaglia-Mayer et al. 2001, 2005; Marzocchi et al. 2008; Scherberger et al. 2003; Snyder et al. 2006). If the monkey fixated outside this window, the trial aborted immediately.
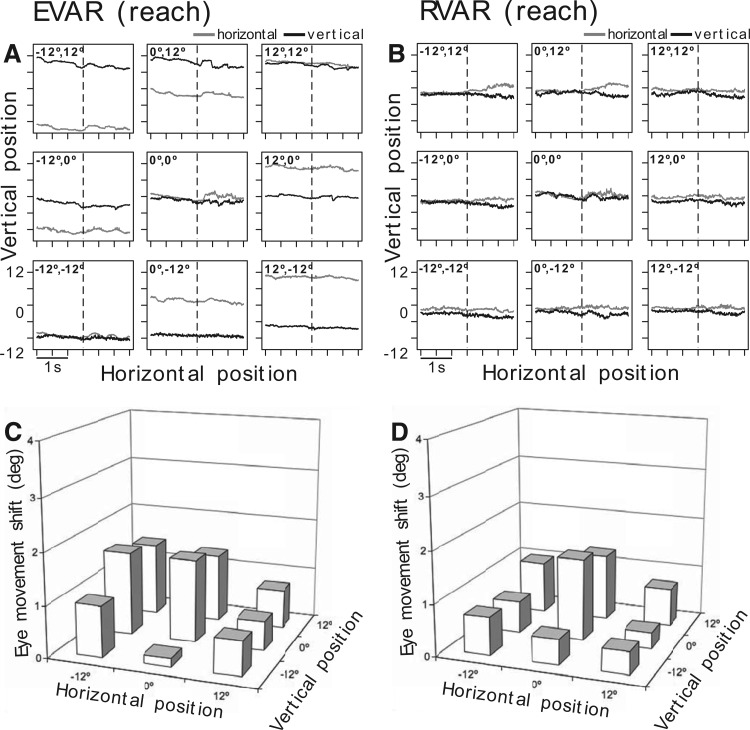
The eye movements were examined off-line at two time points (stimulus onset and lift hand) in the two tasks. One experiment shows typical eye movement traces for two task conditions (Fig. 3). The average eye position traces (10 trials) at the lift hand event for the nine reaching locations are plotted. Although fixation was not as precise as in pure fixation tasks (Andersen et al. 1985, 1990a; Goldberg et al. 2002; Siegel and Read 1997), we could find no bias with different stimulus presentations, hence the larger 4° fixation window. In the EVAR task, no eye movements were associated with the different fixation positions at the lift hand event (Fig. 3A). Most importantly, in the RVAR task, there was no biased eye movement toward the optic flow target at the time of hand lift (Fig. 3B). The average horizontal and vertical eye position from each experiment were transformed into vectors to quantify these effects (Fig. 3, C and D). The difference in vector amplitude 500 ms before and after lift hand was computed. In the example shown, eye position shifts averaged 1° for the EVAR task and 0.8° for the RVAR task. In comparison, eye position shifts at the onset of the optic flow stimulus were on average 2.2° in the EVAR and 1.5° in the RVAR task. These findings were similar across experiments. Thus changes in eye position at the time of the reach event were minimal and could not be responsible for differences in neural activity between tasks.
Fig. 3.
Eye movements before and after reach movement for both EVAR and RVAR tasks in 1 experiment (MFR260.C01). A and B: horizontal (gray) and vertical (black) eye position traces aligned to the reach event (“lift hand event,” dashed line) showing 1.5 s before and after. The 9 panels (3 × 3) for EVAR (A) and RVAR (B) tasks represent 1 typical experimental trial for each position (position in degree visual angle indicated within each panel). C and D: mean deviation (eye position 500 ms before event subtracted from 500 ms after event) averaged over 10 repetitions per position for EVAR (C) and RVAR (D) tasks.
Neural recordings
Neural responses were recorded extracellularly with platinum-iridium electrodes (UEPSEGSG2N5G, FHC, Bowdoinham, ME) with an impedance of 0.5–2.5 MΩ. Penetrations were oriented orthogonal to the cortical surface. The electrode was advanced using a hydraulic motor drive (David Kopf Instruments, Tujunga, CA). After passing through the dura, the depth of the first occurrence of neural activity was recorded, and recordings were confined to 2,000 μm from the top of neural activity. We also recorded activity when retracting the electrode to confirm depth measurements in case of dimpling. Average depth of all recordings was 1,140 ± 565 (SD) μm (n = 155). Per penetration, about one to five units were recorded. Because the sulcal pattern of the PPC was visible through the transparent artificial dura, the two cortical regions of interest could be located without killing the animal (Fig. 1). Area 7a was operationally defined as the cortex between the intraparietal sulcus (IPS) and the end of the superior temporal sulcus (STS) in agreement with earlier studies (Heider et al. 2005; Siegel et al. 2003). DP was defined as the region between the STS and the lunate sulcus (LS).
Neural activity was amplified (Model 1800 Microelectrode AC Amplifier, A-M Systems, Carlsborg, WA), fed through a Humbug 50/60 Hz noise eliminator (AutoMate Scientific, Berkeley, CA), band-pass filtered (300–20,000 Hz), digitized at 40 KHz, and spikes separated off-line with spike sorting software (Off-line Sorter 1.39, Plexon, Dallas, TX). All subsequent quantitative analyses were done on the off-line sorted data. Units were isolated on-line with a dual-window discriminator (BAK, Germantown, MD) to assess neural selectivity during recording.
To obtain a sample as unbiased as possible, activity from all neurons that could be sufficiently isolated were recorded regardless of whether a neuron displayed a preference for the visual or reach aspect of the task. In about one half of all units recorded, preference for type of motion (expansion, compression, and 2 rotational flows) was tested with a centrally presented large field optic flow patch (20° diam). Most area 7a or DP neurons responded robustly to either expansion or compression optic flows as reported previously (Merchant et al. 2001; Read and Siegel 1997; Siegel and Read 1997); thus these two types of stimulus were used for the majority of units. In instances where visual preference for type of optic flow could not clearly be established from the on-line neural response, expansion flow was used.
Spike analyses
Analog data were analyzed off-line with the Plexon software, and spikes were identified by comparing waveforms over the course of the experiment in conjunction with Khoros (Khoral Research, Albuquerque, NM) and custom UNIX-based software. Spike rasters were synchronized to different task events (Fig. 2C, dashed lines), and the firing rate for the epochs of interest was calculated (Anderson and Siegel 1999; Siegel and Read 1997): 1) baseline activity during fixation before stimulus onset; 2) after stimulus onset to determine visual responsiveness (visual epoch); 3) before cue to lift (i.e., stimulus change) to determine movement preparation activity before the reach movement (preparatory epoch); and 4) after lift hand from starting panel to determine reach initiation activity (reach epoch). For the baseline, visual, and preparatory epochs, 500-ms intervals were used. For the reach epoch, a 300-ms interval was used to avoid contamination from the tactile or visual cues when the hand touched the screen.
Categorical regressions
To directly quantify the spatial relationship between the different epochs on a neuron-by-neuron basis, a multiple step procedure was followed. The individual firing rates for all four epochs was first computed. To simultaneously examine the dependency of firing rate on type of epoch and reach target position, a stepwise categorical quadratic regression model was applied. The model for the firing rate was
A(x,y,E,i) is the firing rate for the ith trial. E is the epoch under consideration, which has four categorical values, corresponding to the baseline, visual, preparation, and reach epochs. x and y are the positions of the target on the screen in degrees of visual arc. ax[E]x refers to the four categorical coefficients for the linear dependence on the horizontal position. axx[E]x2 refers to the four possible categorical coefficients for the second-order dependence on the horizontal position. Similar terms are found for the vertical ay[E]y and ayy[E]y2 coefficients and the interaction term axy[E]xy. b[E] is the intercept that can differ for the four categorical epochs. εi is the error for the ith trial. The coefficients (e.g., ax[E]x) were selected using a stepwise algorithm that determined whether the quartet of coefficients was significantly different from zero. At the conclusion of the stepwise algorithm, only coefficients remained that were statistically significant from zero at P < 0.05. The categorical regression was implemented using procedures GLMOD and REG (SAS Institute, Cary, NC). This analysis thus concluded with values that were significant at P < 0.05.
TIME-DEPENDENT TUNING.
The combination of significant regression parameters determined the tuning type for each neuron. Neurons with uniform firing rates across target positions (P) but different mean firing rates across epochs (E) were termed type E. Neurons with significantly different spatial parameters (ax, ay, axy, axx, ayy) across epochs have multiplicative interactions between position and epoch and were termed multiplicative type E×P. These cell response fields changed shape between epochs, that is, their eye position (EVAR task) or retinotopic (RVAR task) spatial tuning was altered as the task progressed. Finally, there were nonsignificant cells with neither epoch nor position effects (NS). Two other tuning types could potentially result from the categorical regression analysis but were not encountered: 1) cells that had at least one significant spatial parameter and maintained the same firing rate between epochs (type P) and 2) cells with at least one significant spatial parameter, which remained constant across epochs, and significant changes in overall firing rate between epochs (E+P).
TASK-DEPENDENT TUNING.
To examine the effects of task on neural reaching activity, categorical regressions examined the effect of task (T, RVAR vs. EVAR) and position (P) separately during the preparatory and reach epochs, because they represent crucial stages of the reaching task. This analysis again showed three different interaction types. Neurons with uniform firing rates across positions but different mean firing rates between tasks were called type T. Neurons that changed spatial parameters between tasks (multiplicative type T×P) had response fields that differed significantly between tasks. Nonsignificant cells (type NS) that had neither task nor position effects were also found. Again, there were no pure position cells (type P) nor were there any additive cells (type T+P).
Both the time-dependent and the task-dependent population spatial parameters were computed for the multiplicative types. For cells with significant linear horizontal and vertical coefficients (ax, ay), the direction of spatial tuning was summarized by the x- and y-vectors. The population spatial parameters were assessed with circular statistics. First, the resulting vectors were transformed into polar coordinates, that is, θ = arctan (ay/ax) with the convention of 0° (360°) corresponding to the right position “East” (12, 0°) with a counterclockwise rotation. For the population of vectors, the Hotelling one-sample test (Batschelet 1981; Zar 1984) determined whether vectors were distributed nonuniformly. This statistic uses the amplitude and the direction of the angular tuning. Therefore a weakly spatially tuned cell (i.e., with small linear coefficients) contributes less to the statistic than a strongly tuned cell for a given direction. Significance level for the resulting F-test was set at P < 0.05. To examine changes in spatial tuning, the directions of the difference vectors (axprep − axvis, ayprep − ayvis) were computed between the preparatory and visual epoch. Such a comparison shows modulation early and late in the delay period and thus reflects increasing influences from nonvisual signals.
In cells with mixed linear and quadratic coefficients, the dimensions of the resulting response fields were determined based on the combination of significant parameters (ax, ay, axx, ayy), similar to previous studies (Heider et al. 2005; Quraishi et al. 2007). Cells that had a quadratic dependence along both the horizontal and vertical had a local maximum (“peak,” negative axx and ayy terms), a local minimum (“trough,” positive axx and ayy terms), or a “saddle” shaped response field (different signs for axx and ayy terms). For the two latter combinations, the highest firing rate is located in the periphery of visual space. Thus for cells with quadratic coefficients, the preferred direction (maximum response) can only be established for cells with negative quadratic coefficients (peaks) and without interaction parameters (axy). To visualize the spatial tuning for each significant cell, response fields derived from the quadratic, and linear coefficients were created.
To determine whether neural responses differed between two epochs (visual vs. preparatory) on a trial-by-trial basis, the firing rate was computed for the two epochs averaged across all nine positions. The averaged firing rate was compared with a paired t-test generating a probability value, P. The number of neurons with a probability of P < 0.05 was calculated. This analysis is called paired analysis throughout the paper. For comparison of cell type distributions, a χ2 test was used with a significance level of P < 0.05.
RESULTS
Behavioral data
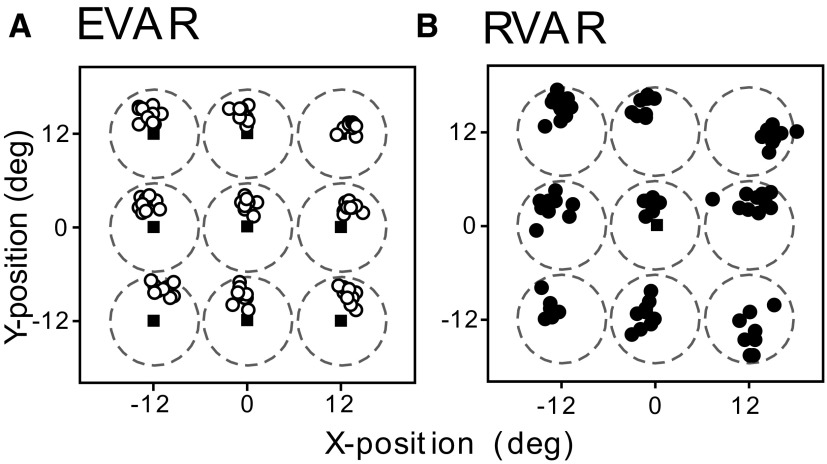
Behavioral measurements consisted of reach endpoint accuracy relative to the center of the target and response times. Endpoints on the screen from one experiment (EVAR and RVAR) are plotted in Fig. 4. This example shows that reaching was more accurate (i.e., closer to the target center) in the EVAR than RVAR task. A paired comparison of the vectors derived from the horizontal and vertical endpoint positions confirmed that accuracy was indeed greater in the EVAR task when the reach target was foveated (t-test, P = 0.007). The lower endpoint accuracy in the RVAR task may be the result of the larger difference between reach endpoint and fovea and the known gaze-dependent errors when pointing to peripheral targets (Henriques and Crawford 2000).
Fig. 4.
Reach endpoints of 1 typical experiment (n = 90 trials, MFR256.C01) during the EVAR task (A, ○) and the RVAR task (B, ●). Dashed circles indicate circumference of optic flow patches (diameter 12°).
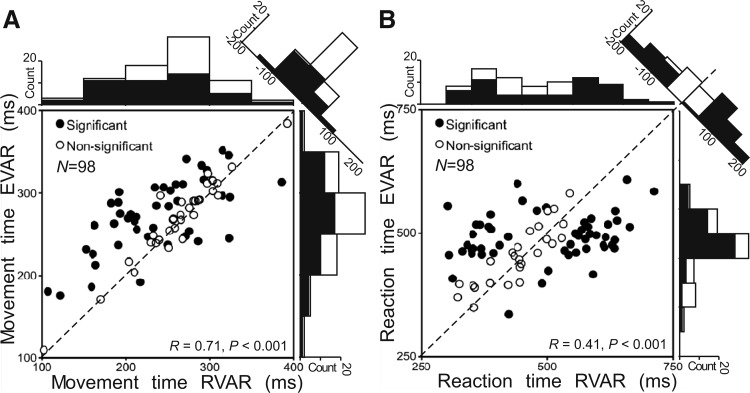
Two behavioral time segments were computed for all experiments: reaction time (RT), that is, the period from the change in optic flow to the hand lift, and movement time (MT) from the hand lift to the moment the hand touched the screen. These two time segments were compared between the RVAR and EVAR task for 98 recordings where both tasks were completed (Fig. 5). Within an experimental recording, MTs from start panel to touch screen were significantly shorter in the RVAR task (t-test, P < 0.001) than in the EVAR task (Fig. 5A).
Fig. 5.
Distribution and scatter plots of mean behavioral times (T) for the 2 tasks (TEVAR: EVAR; TRVAR: RVAR). ●, behavioral times that were significantly different (TEVAR vs. TRVAR) with Student's t-test; ○, nonsignificant values. Along each axis, histograms show distribution of behavioral times for the significant (filled bars) and nonsignificant (open bars) values. The angled histograms represent TEVAR − TRVAR. A: movement times (time from hand lift to touch). B: reaction times (time from stimulus change to hand lift). Each dot represents the mean of the behavioral times during 1 recording run (90 trials).
For the RTs (Fig. 5B), there were no significant differences between the EVAR and the RVAR tasks with respect to the mean. The pairwise plots examined how these behavioral parameters varied on a day-by-day basis. Both movement times and reaction times varied between experiments but were significantly correlated between tasks within a day (Fig. 5, A and B). Some days, the monkey responded very fast, whereas on other days, reaction and movement times were slower in both tasks. The two animals differed in their mean detection and travel times but showed the same task effects.
Across all experiments, the average reach velocity was ∼1.2 m/s, which lies well within published data for fast, ballistic reaching (Churchland et al. 2006; Gardner et al. 2007; Kurata and Hoshi 2002). These behavioral reach data were obtained largely without visual feedback (Desmurget and Grafton 2000; Vercher et al. 1994), because the monkey could not see his hand until it reached the touch screen. The slower speed (i.e., longer movements times) found when reaching to foveated targets in the EVAR task matches published studies in humans (Prablanc et al. 1986). These behavioral differences between the two task conditions suggest different computational strategies and have to be considered when interpreting the single unit data (Moran and Schwartz 1999; Snyder et al. 2006).
Electrophysiological dataset
The main goal of this study was to examine the spatial relationship between baseline, visual, preparatory, and reach signals in area 7a and DP. The statistical analyses thus focused on temporal (epochs), spatial (reach target position), and contextual (task condition) aspects of the neural response. A total of 164 units (90 in M1R, 74 in M3R; 99 in area 7a, 65 in DP) were studied quantitatively using the EVAR task. For the RVAR task, 119 units were studied (62 in M1R, 57 in M3R; 65 in area 7a, 54 in DP). For a total of 98 cells, both the RVAR and EVAR tasks were completed (53 in 7a; 45 in DP).
The data are presented three ways. First, the epoch-based analysis and modeled response fields are exemplified for one area 7a and one DP cell. Second, the epoch-based analysis examines temporal and spatial aspects within the EVAR and RVAR tasks for the population of neurons. Third, the EVAR and RVAR tasks are compared directly during preparatory and reach epochs to show the effect of eye position.
Single unit activity synchronized to task epochs
EVAR TASK.
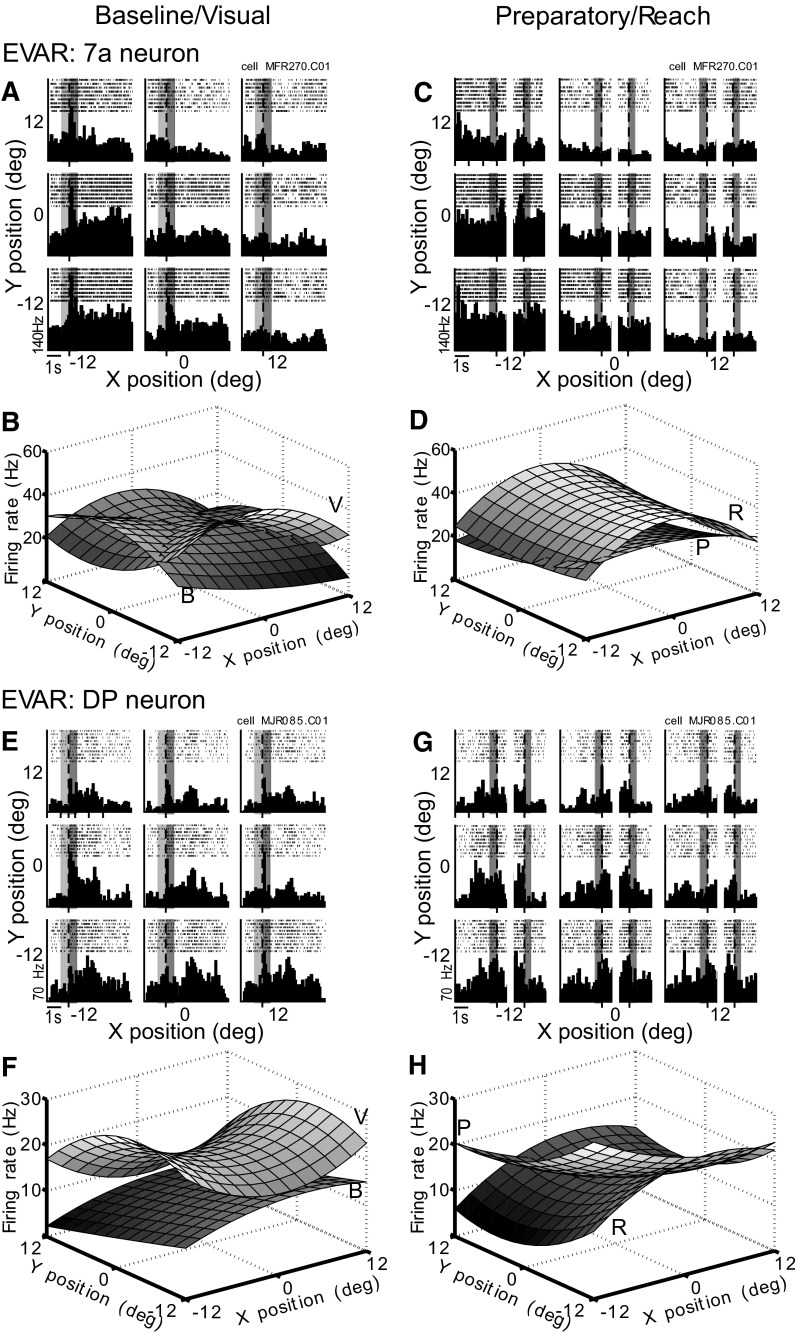
The 7a neuron (Fig. 6, A–D) responded strongly during all epochs in the EVAR task. The baseline activity before stimulus onset was strongest for the contralateral (left) eye positions (Fig. 6A, light gray–shaded bars). The neuron responded with a transient burst of activity to the onset of the visual stimulus for the contralateral and lower eye positions (Fig. 6A, dark gray–shaded bars). The baseline and visual activity resulted in two differently shaped response fields (Fig. 6B). During the preparatory epoch, the spatial preference remained constant, with higher activity for the contralateral and lower eye positions (Fig. 6, C and D). After the lift hand event, the spatial preference shifted upward toward the midline (Fig. 6, C and D).
Fig. 6.
EVAR task. A–D: area 7a neuron (MFR270.C01, expansion optic flow). A: spike rasters and peristimulus time histograms (PSTHs) for the 9 reach targets aligned to stimulus onset event (baseline and visual epochs, light-shaded and dark-shaded regions, respectively). Each subpanel of the 3 × 3 panels represents neural responses for 1 target location. Bin width, 50 ms. B: quantitative modeled response fields for baseline (B) and visual epochs (V). Equations are for firing rate in hertz. Abase = −0.71x + 0.21y − 0.021x2 − 0.078y2 + 27.4. Avis = −0.46x − 0.76y − 0.115x2 + 0.074y2 + 30.5. C: spike rasters and PSTHs aligned to reach cue event (preparatory epoch, dark-shaded region, left half of each subpanel) and lift hand event (reach epoch, dark-shaded region, right half of each subpanel). D: response fields for preparatory (P) and reach (R) epochs. Aprep = −0.26x − 0.61y − 0.036x2 + 0.023y2 + 24.2. Areach = −0.12x − 0.094y − 0.132x2 + 0.019y2 + 40.8. E–H: area DP neuron (MJR085.C01, expansion optic flow). E: rasters and PSTHs aligned to stimulus onset. F: response fields. Abase = 0.17x − 0.37y − 0.02x2 − 0.001y2 + 11.5. Avis = −0.12x − 0.39y + 0.051x2 − 0.05y2 + 20.1. G: rasters and PSTHs aligned to reach cue and lift hand. H: response fields. Aprep = −0.44x − 0.58y + 0.023x2 + 0.03y2 + 14.5. Areach = 0.31x − 0.37y − 0.042x2 + 0.037y2 + 14.8.
The DP neuron (Fig. 6, E–H) had a weak spatial tuning during the baseline fixation epoch with elevated activity for the lower ipsilateral (right) eye positions (Fig. 6E). Steep increases in activity were observed at stimulus onset for the contralateral and ipsilateral eye positions (Fig. 6E), resulting in a saddle shaped response field (Fig. 6F). This eye position tuning changed during the preparatory epoch (Fig. 6G) with a preference for lower contralateral targets. During the reach epoch, firing increased further for the lower center position (Fig. 6G) and changed the shape of the response field (Fig. 6H).
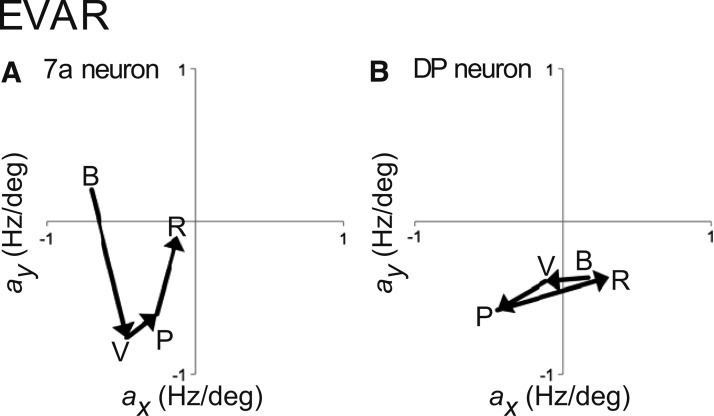
The spatial tuning across time for both neurons is shown by plotting the linear coefficients (Fig. 7). The 7a neuron (Fig. 7A) started with an upper contralateral eye position tuning during baseline (B) and shifted to a lower contralateral tuning when the stimulus appeared (V). During the preparatory epoch, this tuning moved toward the vertical midline (P) and finally upward toward the center after the monkey launched the reaching movement (R). The DP neuron (Fig. 7B) shifted eye position tuning from lower ipsilateral (B) to contralateral (V, P) and back to the lower ipsilateral eye positions (R) as the task progressed.
Fig. 7.
Changes in spatial eye position tuning (EVAR) for the example area 7a (A) and DP (B) neurons (see Fig. 6). Black arrows indicate shifts in spatial preference (linear coefficients) between baseline, visual, preparatory, and reach epochs.
RVAR TASK.
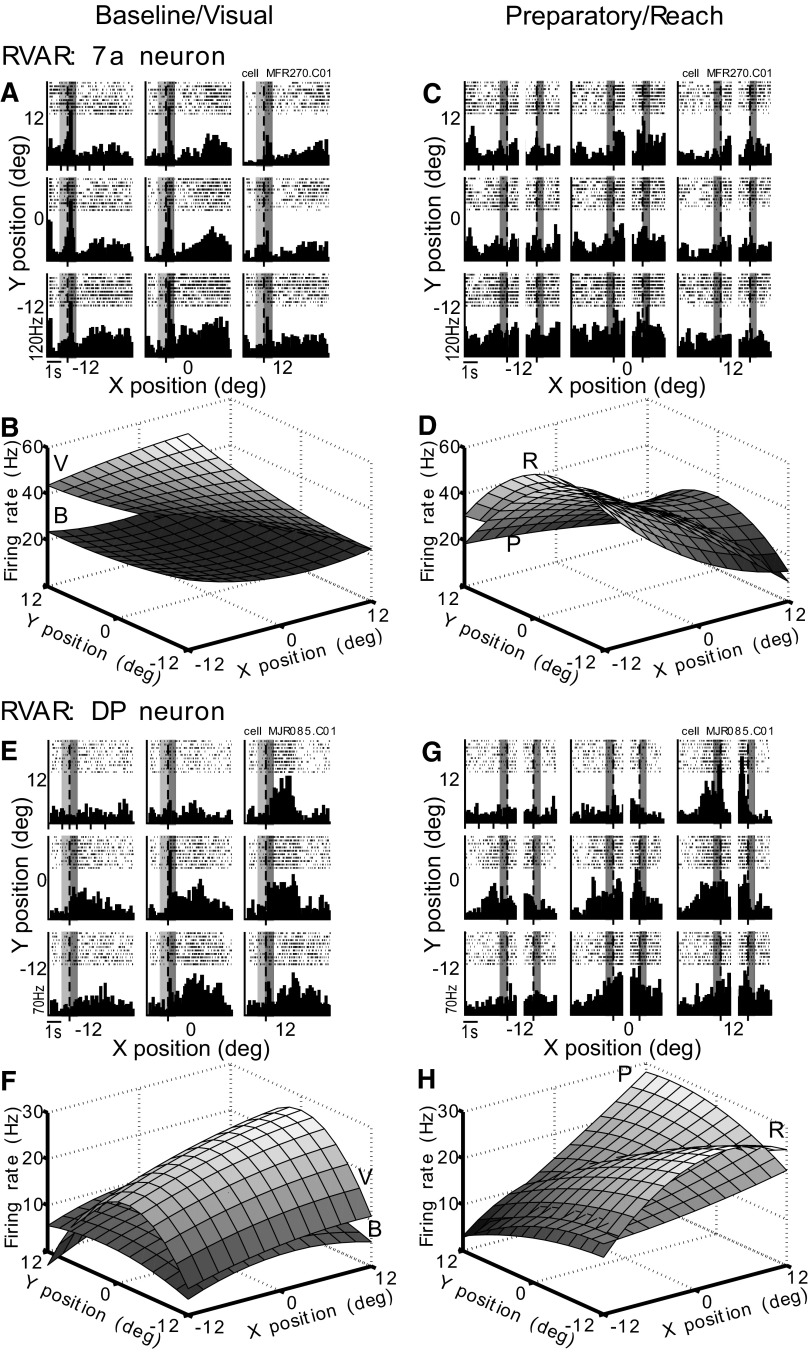
The 7a neuron exhibited a distinct retinotopic tuning when fixation was kept constant on the center position (Fig. 8, A–D). Baseline tuning was flat (Fig. 8, A and B) as expected. At stimulus onset, the neuron fired for targets appearing in the lower contralateral visual field (Fig. 8A), resulting in a tilted response field (Fig. 8B). During the preparatory epoch, the retinotopic tuning remained in the lower contralateral visual field (Fig. 8, C and D) and then moved further upward toward the midline (Fig. 8, C and D) during the reach epoch.
Fig. 8.
RVAR task. A–D: area 7a neuron (MFR270.C01); conventions as in Fig. 6. A: spike rasters and PSTHs aligned to stimulus onset. B: response fields. Abase = −0.061x − 0.18y + 0.079x2 + 0.025y2 + 38.6.4. Avis = −2.27x − 1.4y + 0.026x2 − 0.007y2 + 38.6. C: spike rasters and PSTHs aligned to reach cue and lift hand. D: response fields. Aprep = −1.46x − 1.05y − 0.01x2 − 0.082y2 + 38.6. Areach = −1.29x − 0.56y − 0.057x2 − 0.12y2 + 38.6. E–H: area DP neuron (MJR085.C01). E: rasters and PSTHs aligned to stimulus onset. F: response fields. Abase = 0.077x − 0.09y − 0.024x2 − 0.018y2 + 11.4. Avis = 0.39x − 0.19y − 0.011x2 − 0.113y2 + 23.8. G: rasters and PSTHs aligned to reach cue and lift hand. H: response fields. Aprep = 0.64x − 0.1y + 0.006x2 − 0.021y2 + 19. Areach = 0.26x − 0.65y − 0.06x2 − 0.013y2 + 21.3.
The DP neuron's response field was flat during the baseline period (Fig. 8, E and F). Stimuli appearing in the center and ipsilateral visual field yielded increased neural activity (Fig. 8E), resulting in a peaked response field for the visual epoch (Fig. 8F). The preparatory epoch was characterized by strongly elevated activity for the upper ipsilateral retinotopic targets yielding a steeply tilted response field (Fig. 8, G and H). After the lift hand event, the firing rate sharply decreased, especially for those positions that showed peak firing during the preparatory epoch (Fig. 8G). During the reach epoch, the highest firing rates were seen for the lower ipsilateral targets (Fig. 8, G and H).
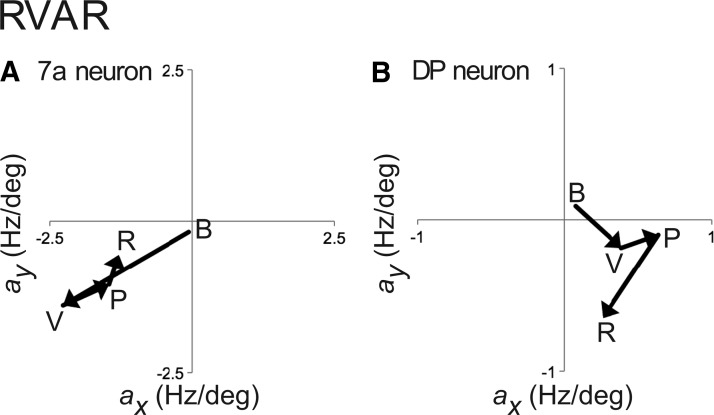
The corresponding plot of the linear coefficients for the 7a neuron during the RVAR task (Fig. 9A) shows how the cell's spatial preference started in the center during baseline (B) and moved toward the lower contralateral visual field after stimulus onset (V). During preparation (P) and initiation of the reach (R), the retinotopic spatial tuning shifted back toward the center. The DP neuron (Fig. 9B) switched its retinotopic spatial tuning from center (B) toward the ipsilateral visual field at stimulus onset (V), further ipsilateral during preparation (P), and downward during reach (R).
Fig. 9.
Changes in spatial retinotopic tuning (RVAR) for the example area 7a (A) and DP (B) neurons (see Fig. 8). Conventions as in Fig. 7.
Comparison of spatial tuning between epochs
These two example neurons show the complex interactions between reach target location and epoch. Area 7a and DP neurons altered their spatial eye position and retinotopic tuning during the task. To quantify such changes for the population of recorded neurons, different tuning types were determined using the results of the categorical regression. Cells were classified as 1) having significant changes in overall firing rate without spatial tuning (type E), 2) having time-variant spatial tuning (type E×P), or 3) cells without effects (type NS).
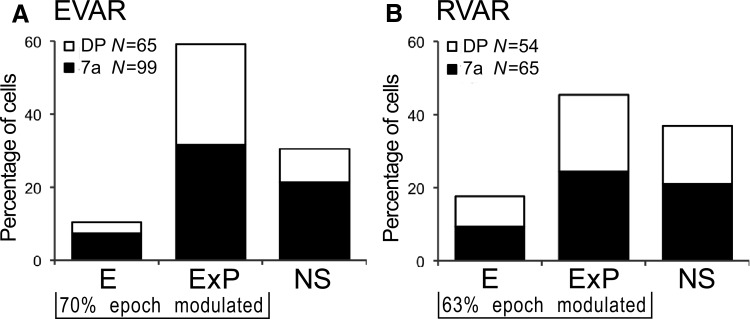
EVAR task.
The majority of cells (70%, 114/164) showed an effect of epoch and/or position as quantified by the statistical categorical regression (Fig. 10A). The distribution of the two tuning types (E, E×P) did not differ significantly between areas. Area 7a and DP also contained neurons that did not respond during any of the selected epochs (NS, total: 30%, 50/164; 7a: 35%, 35/99; DP: 23%, 15/65). The type E neurons showed flat spatial tuning and significant differences in intercepts between at least two epochs (total: 10%, 17/164; 7a: 12%, 12/99; DP: 8%, 5/65). Comparison of intercepts between visual and preparatory epochs did not show significant differences.
Fig. 10.
Proportions of interaction type between epoch (E) and position (P) plotted separately for each area (7a, filled bars; DP, open bars) during EVAR (A) and RVAR (B) task conditions. E-type neurons had a single effect of epoch (change in mean firing rate) but were not spatially tuned. E×P-type neurons (multiplicative interaction) had different spatial tuning between epochs. NS cells had no effect of either factor.
The multiplicative (type E×P) neurons comprised 59% of the cells (total: 97/164; 7a: 52%, 52/99, DP: 69%, 45/65). These cells were fit with at least one significant spatial parameter (ax, ay, axy, axx, ayy) during at least two of the four epochs. Of these 97 E×P cells, 85 had at least two significant spatial parameters for the visual, preparatory, and/or reach epochs. Forty-one of the 97 E×P cells (42%) were only linearly modulated by the stimulus position. In these cases, the horizontal (ax) and vertical (ay) terms indicate the maximum response for the preferred eye position. For all epochs, the angular eye position tuning was distributed uniformly for this population of E×P neurons. A uniform distribution was also present for the vector of the spatial shifts between visual and preparatory epochs. Another 36% (35/97) of E×P neurons had an additional quadratic dependence in at least one dimension. Significant interaction terms were present in 21% (20/97) of the E×P neurons.
The differences in firing rate between visual and preparatory epochs were established using the paired analysis (see methods), which computed the change in activity between preparatory and visual epoch for each E×P neuron. For this group, 43% of neurons (7a: 22/97; DP: 20/97) had significantly different firing rates. During these two epochs, the monkey was maintaining fixation at one location, the visual stimulus remained constant (i.e., structured optic flow), and the hand was resting on the start panel. Thus changes in neural activity can be attributed only to internal processing (e.g., motor planning, attention).
RVAR task.
With the eye position kept constant at the primary position (0, 0°), about two thirds (63%, 75/119) of the cells had an effect of epoch and/or position (Fig. 10B). The distribution of the two tuning types (E, E×P) was similar between areas. Area 7a and DP also contained neurons that did not respond during any of the selected epochs (NS, total: 37%, 44/119; 7a: 38%, 25/65; DP: 35%, 19/54). The type E neurons were modulated by the event but had no spatial retinotopic tuning (total: 18%, 21/119; 7a: 17% 11/65; DP: 19% 10/54). Intercepts for the visual epoch were significantly higher than for the preparatory epoch (t-test, P = 0.006), suggesting that having retinotopically varied stimuli yielded high firing rates immediately after stimulus onset, which decreased substantially toward cue event. These type E cell receptive fields could be large and bilateral as reported previously (Motter and Mountcastle 1981; Mountcastle et al. 1987), therefore not showing significant spatial modulation from the limited display size (36 × 36°).
The multiplicative (type E×P) neurons comprised 45% (total: 54/119; 7a: 45%, 29/65; DP: 46%, 25/54) of the cells. Pure linear modulation was present in 30% (16/54), whereas 33% (18/54) of E×P neurons had an additional quadratic dependence. For population of purely linear cells, angular tuning was uniformly distributed. Significant interaction terms were present in 22% (12/54) of the E×P neurons. Among the 54 E×P cells, 43 had significant spatial parameters for the visual, preparatory, and/or reach epoch. With the paired analysis, it was found that 52% of the E×P neurons (7a: 14/54; DP: 14/54) significantly changed their firing between the visual and preparatory epoch.
In summary, the regression analysis provided a robust quantitative measure of the differential effects of epoch on a neuron-by-neuron basis during the EVAR and RVAR tasks. About two thirds of the cells were altered by epoch as the task progressed, more often in form of changes in spatial tuning than in form of gain changes. Although the stimulus on the screen provided constant visual stimulation throughout the task, additional spatially tuned inputs related to the preparatory and reach event impacted these neurons' activity.
Comparison of single unit activity between RVAR and EVAR task
Neural activity during the preparatory and reach epochs was directly compared on a neuron-by-neuron basis for conditions where the location of the reach targets was identical, but reach movements were made under different combinations of eye and retinal information. In the EVAR task, the monkey reached to a foveated target; thus eye position varied concurrently with reach target position, whereas the retinotopic stimulation remained constant on the fovea. In the RVAR task, the target was not foveated (except for the center position) and the retinotopic stimulation varied. A subset of 98 cells was analyzed in which both the EVAR and RVAR tasks were tested. Categorical regressions determined the effects between the two factors: task condition (T, EVAR vs. RVAR) and position (P, 9 reach target positions). These effects were determined separately for each of the four epochs, but the focus will be on the preparatory and reach epochs. Neurons were grouped according to whether they changed their tuning in the task or spatial domain.
BASELINE AND VISUAL EPOCHS.
Firing rate for the center position was compared between EVAR and RVAR tasks to identify possible contributions from attentional or other effects between the two task blocks. For both epochs, firing rates did not differ significantly between the two tasks.
PREPARATORY EPOCH.
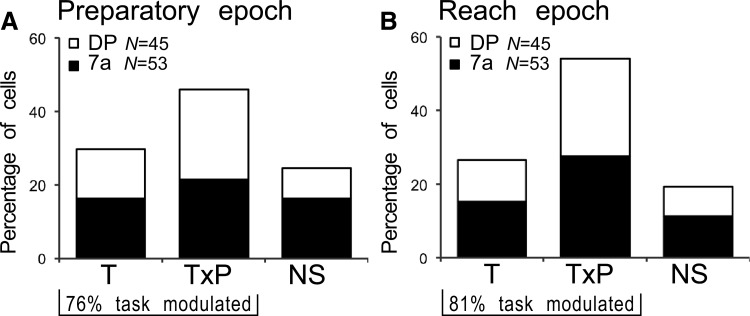
This epoch is characterized by an absence of sensory or motor changes. The monkey is fixating, a structured optic flow stimulus is displayed, and the hand is resting on the launching panel. During this epoch, the task factor affected 76% (74/98) of the cells significantly (Fig. 11A), suggesting that the majority of cells changed their tuning or gain depending on whether the hand was going to be moved to a foveated or peripheral reach target. The two tuning types (T, T×P) were distributed evenly between area 7a and DP. About one quarter of the neurons were not affected by either factor (NS, total: 24%, 24/98; 7a: 30%, 16/53; DP: 18%, 8/45). The type T was observed in 30% of the cells (29/98; 7a: 30%, 16/53; DP: 29%, 13/45). These cells differed in their mean firing rates between RVAR and EVAR conditions but had flat spatial tuning. Intercepts did not differ significantly between the two conditions for this group.
Fig. 11.
Proportions of interaction type between task condition (T) and position (P) plotted separately for each area (7a, filled bars; DP, open bars) for the preparatory epoch (A) and the reach epoch (B). T-type neurons had different firing rates between tasks and flat spatial tuning. T×P-type neurons (multiplicative interaction) changed spatial tuning (response fields) between tasks. NS cells had no effect of either factor.
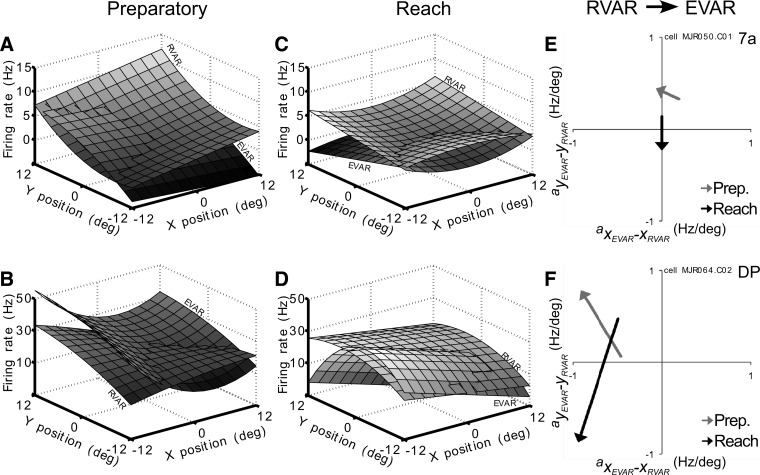
The multiplicative type T×P was present in 46% of cells (total: 45/98; 7a: 40%, 21/53; DP: 53%, 24/45). In this group of neurons, identical reach targets yielded different spatial tuning during the preparation of the reaching movement. Typical response fields of 7a cells are shown in Fig. 12A. This cell showed peak firing for upper ipsilateral retinotopic targets (positive vertical and horizontal coefficients) during the RVAR task. When reaching was performed to foveated targets (EVAR), spatial preference shifted toward eye positions along vertical midline (Fig. 12E, gray arrow). Another DP cell example fired mostly for targets in the contralateral hemispace during RVAR condition and moved its preference toward the upper contralateral space during EVAR condition (Fig. 12, B and F, gray arrow).
Fig. 12.
Response fields and spatial tuning of a typical area 7a (MJR050.C01; A, C, and E) and a DP neuron (MJR064.C02; B, D, and F). Preparatory epoch during EVAR and RVAR task (A and B): MJR050.C01 AEVAR = −0.068x + 0.45y + 0.003xy − 0.011y2 + 2.53; ARVAR = 0.19x + 0.33y + 0.024xy − 0.021y2 + 2.53. MJR064.C02 AEVAR = −0.91x + 0.79y − 0.004xy − 0.12x2 + 21; ARVAR = −0.46x + 0.06y − 0.072xy − 0.012x2 + 21. Reach epoch during EVAR and RVAR task (C and D). MJR050.C01 AEVAR = −0.24y − 0.009x2 + 1.78; ARVAR = 0.14y + 0.019x2 + 1.78. MJR064.C02 AEVAR = −0.98x − 0.88y − 0.18y2 + 22.4; ARVAR = −0.49x + 0.47y − 0.057y2 + 22.4. Corresponding shifts in spatial preference (linear coefficients) between RVAR and EVAR tasks for preparatory (gray arrows) and reach (black arrows) epochs (E and F).
Similar to the analysis of the E×P cells, the T×P cells during the preparatory epoch were fit with at least one significant spatial parameter (ax, ai, axy, axx, ayy) during both tasks. Sixteen of these 45 cells (36%) were only linearly modulated by the target position. Angular tuning distribution for these purely linear T×P cells was uniform. Directions of spatial shifts between linear parameters for the EVAR and RVAR conditions were also uniform. Another 40% of T×P neurons (18/45) had an additional quadratic dependence in at least one dimension. Significant interaction terms were present in 27% (12/45).
REACH EPOCH.
This epoch differed from the preparatory epoch by the additional sensory and motor changes (visual stimulus change and the onset of hand movement initiation). The majority of the cells (total: 81%, 79/98; 7a: 79%, 42/53; DP: 82%, 37/45) showed significant task effects (Fig. 11B), confirming the hypothesis that the activity during movement initiation was strongly affected by the task condition, that is, whether the monkey was going to reach to a foveally or eccentrically presented stimulus. There were no significant differences in distribution of the T and T×P types between the two areas. Nonsignificant cells comprised 19% of the population (NS, total: 19/98; 7a: 21%, 11/53; DP: 18%, 8/45). About one quarter of the cells were type T neurons (total: 27% 26/98; 7a: 28%, 15/53; DP: 24%, 11/45). Intercepts between RVAR and EVAR conditions for this group did not differ significantly.
More than one half of the cells were of the multiplicative type T×P (total: 54%, 53/98; 7a: 51%, 27/53; DP: 58%, 26/45). Thus identical reach target positions yielded varying spatial preferences depending on whether the target was foveated or not. The area 7a cell changed spatial preference from upper to lower contralateral reach positions between EVAR and RVAR task (Fig. 12, C and E, black arrow). The DP cell shifted preference from upper to lower reach positions between RVAR and EVAR tasks (Fig. 12, D and F, black arrow). Seventeen of these 53 cells (32%) were only linearly modulated by the target position and had a uniform distribution of spatial tuning. Spatial shifts between EVAR and RVAR conditions were also distributed uniformly. Another 38% of the T×P neurons (20/53) had an additional quadratic dependence in at least one axis. Significant interaction terms were present in 28% (15/53) of the T×P neurons.
DISCUSSION
Combined findings from various studies have lead to the general consensus that PPC neurons are involved in goal-directed behaviors including attentional and motor planning signals (Andersen et al. 1997; Burnod et al. 1999; Coulthard et al. 2008; Rozzi et al. 2008; Snyder et al. 2006). This study builds on prior reaching studies in area 7a (Battaglia-Mayer et al. 2005, 2007; Blum 1985; MacKay 1992; Mountcastle et al. 1975; Rushworth et al. 1997) and expands our knowledge about PPC neurons, including DP. First, spatial tuning was quantitatively compared between the baseline, visual, preparation, and initiation phases of the reaching task. Modeled response fields show the changes in spatial tuning over time. Second, area 7a and DP neurons were classified into subpopulations with distinct spatial and temporal tuning properties. Third, the role of eye position on reaching activity was examined by having the monkeys reach to foveated (EVAR task) or nonfoveated (RVAR task) targets. Fourth, and importantly, DP was identified as containing neurons that are modulated during the planning and initiation phases of the reaching task.
Methodological considerations
In this study, reaching movements occurred in a radial (3D) manner. This differs from a lateral reach, where the monkey starts the reach from a target on the screen and then lifts the hand briefly off the screen to move to the next target on a plane, as used in many reaching studies (Batista and Andersen 2001; Battaglia-Mayer et al. 2005, 2007; Scherberger and Andersen 2007; Snyder et al. 2006). A radial reach more closely resembles a natural movement (MacKay 1992). Because the animal was fixating before making the reach movement, the hand could not be seen until it hit the screen and partially occluded the optic flow target.
Neural responses during different epochs
About one half of the neurons responded to the onset of the visual stimulus and showed effects of eye and retinal position, as demonstrated previously (Battaglia-Mayer et al. 2007; Fischer and Boch 1981a,b; Maguire and Baizer 1984; Merchant et al. 2001; Mountcastle et al. 1987; Read and Siegel 1997; Siegel and Read 1997; Youakim et al. 2001). Within each task condition (EVAR or RVAR), we assume that the initial visually dominated response was affected by multiple signals during the preparatory and reach epochs.
PREPARATORY RESPONSES.
The preparatory epoch was operationally defined as the 500 ms before the cue to reach. Changes in neural firing during this delay epoch were not contaminated by overt sensory events or motor behaviors. The visual stimulus was a constant optic flow, and the hand remained at a resting position. As the target appeared on the screen several seconds before the cue to reach, the monkey had enough time to shift his attention and anticipate the upcoming reach cue. It is very likely that the preparation of the reaching movement began as soon as the fixation spot and/or reaching target appeared on the screen. Thus the preparatory epoch likely represents a phase of movement preparation, during which integration of multiple signals can occur.
First, the spatial locus of attention has been shown to alter neural activity in area 7a and DP (Bender and Youakim 2001; Bushnell et al. 1981; Mountcastle et al. 1981; Quraishi et al. 2007; Raffi and Siegel 2005; Steinmetz and Constantinidis 1995). In the EVAR task, attention was overtly directed because the reach target was always foveated, whereas in the RVAR task, attention was covertly directed to the nonfoveated reach target (Bushnell et al. 1981; Quraishi et al. 2007; Steinmetz et al. 1994). Covert and overt attention are likely to modulate neural responses during the RVAR and EVAR conditions, respectively. However, the variety of neural response types suggests that the preparatory modulation went beyond mere attentional enhancement or suppression of the visual signal (Sakata et al. 1995). Recent findings in lateral intraparietal area (LIP) showed that motor planning and attentional processes can be separated anatomically (Liu et al. 2010). Studies in PRR also strongly suggest that motor planning can be distinguished from attentional modulation because it is dependent on the effector (Calton et al. 2002; Snyder et al. 2006).
Second, this study also showed that motor planning likely contributed to the preparatory signal through known anatomical feedforward and feedback pathways (Andersen 1997; Scherberger et al. 2005). It has been proposed that parietal cortex represents an early stage in movement planning (Snyder et al. 2000). Covert movement planning during a memory or delay period modulates neurons in 7a (Snyder et al. 1997). Thus both area 7a and DP form part of a larger neural network that is crucial for transforming visual spatial information into a motor plan (Scherberger and Andersen 2007).
REACH RESPONSES.
The firing rate during the reach epoch likely had both overt sensory and motor contributions, because the monkey detected the stimulus change, initiated the hand movement, and moved his hand toward the target. Changes in spatial tuning could derive from joint proprioception, because primates are quite accurate in localizing their limbs in 3D space even in absence of visual or tactile inputs (Baraduc et al. 2001; Ghika et al. 1995; Kalaska 1988; Prud'homme and Kalaska 1994; Ren et al. 2006; Scheidt et al. 2005). Such inputs are likely to be conveyed through other parietal areas that respond to somatosensory and proprioceptive manipulations (Breveglieri et al. 2006; Fattori et al. 2001; Ferraina et al. 1997, 2001; Johnson et al. 1996). An important contribution to the reach signal could derive from efference copy or corollary discharge (Hyvärinen 1982; Mountcastle et al. 1975; Rushworth et al. 1997). Indirect feedback from motor cortices through areas 7b or 5 might be involved in such signals (Gardner et al. 2007). These multiple signals are combined in time to monitor and modulate ongoing reaching movements (Beurze et al. 2007). These modulatory signals likely propagate to DP through strong reciprocal connections with area 7a and other parietal and frontal areas (Andersen et al. 1990a; Cavada and Goldman-Rakic 1989b; Stepniewska et al. 2005). Thus this population of PPC neurons integrates information from multiple cortical sources to calculate the sensorimotor transformation.
Sensorimotor transformation and classification of neurons
The majority of studied area 7a and DP neurons modulated their firing rate during the baseline, visual, preparatory, and/or reach epochs. Categorical regression methods were used to classify neurons into different tuning types based on interactions between epoch and position. In the EVAR task, a small proportion of neurons (∼10%) were modulated by the epoch but were not spatially tuned (E-type cells). During the RVAR task, a slightly higher proportion of this tuning type was found. Large receptive fields that extend beyond the display could explain nonselectivity to retinotopic position. Overall, cells of this type for could reflect a gain signal from motor planning (Bullock et al. 1998; Snyder et al. 1998) or from nonspatial attentional modulation.
A substantial number of spatially tuned cells changed their spatial preference (eye position tuning or retinotopic tuning) during progression of either task (E×P). During the EVAR conditions, 59% of the neurons started with a certain eye position preference at baseline and stimulus onset and altered their spatial tuning as the monkey prepared and executed his reach movement. In the RVAR task, 45% changed their retinotopic tuning. This suggests a predominance of gain field tuning over retinotopic tuning in the population of neurons, which is in agreement with earlier studies (Heider et al. 2005; Read and Siegel 1997; Siegel et al. 2003). Substantial changes in spatial tuning occurred between the visual and preparatory epoch for both conditions. These changes could arise from spatially tuned attentional or planning signals based on other coordinate frames (e.g., arm-centered, head-centered) originating from frontal or other parietal areas (Pesaran et al. 2006; Rozzi et al. 2006). These signals modulated and changed the eye position and retinotopic tuning that prevailed during the early “visual” phase of the task. Reach-related modulation, when the hand was lifted off the start position, could further be attributed to a combination of sensory (e.g., visual, proprioceptive) and motor signals as suggested for other parietal areas (Breveglieri et al. 2008).
Thus shifts in spatial tuning could represent highly specific feedback from spatially tuned neurons in other cortical areas. Alternatively, initial generation or on-line modification of the motor command could arise within area 7a or DP to guide the ensuing reach movement. Such motor commands need to be generated in conjunction with visual information. This could involve intracortical circuitry within area 7a and/or DP, consistent with lesion and imaging studies that support the role of parietal cortex in on-line adjustment of reaching movements under visual control (Buxbaum and Coslett 1998; Desmurget et al. 1999; Pisella et al. 2000).
Contextual dependence of the sensorimotor transformations
To achieve accurate reaching performance, information from various sources (e.g., visual, proprioceptive, efference copy) is updated continuously, allowing comparison of targeted with actual movement (Desmurget et al. 1998). Previous studies have shown that gaze direction and retinotopic location of a visual target modulate neural activity in both area 7a and DP neurons (Andersen et al. 1985, 1990a; Bremmer et al. 1997; Heider et al. 2005; Read and Siegel 1997; Siegel et al. 2003, 2007). To separate the contributions of retinotopic and gain field signals, the loci of fixation and retinal stimuli were systematically varied and quantitatively assessed.
Behavioral effects of reaching to nonfoveated targets consisted of decreased reaching accuracy and shorter movement times, suggesting involvement of distinct neural populations. This also supports macro-level description of recruitment of different neural networks for foveal versus peripheral reaching assessed with event-related functional MRI and human lesion studies (Clavagnier et al. 2007; Pisella et al. 2009). Behavioral differences between the tasks could also be caused by differences in activity within the same population of neurons. For example, parietal area 5 cells combine signals from multiple sources to encode information about change of movement trajectory (Archambault et al. 2009; Chen et al. 2009).
Human psychophysical findings support a strong coupling between gaze direction and pointing movements (Admiraal et al. 2004; Dijkerman et al. 2006; Henriques et al. 1998; Neggers and Bekkering 2001). When the gaze is held steady on the reach target, efference copy and ocular proprioception allow greater accuracy in reaching (Lewis et al. 1998; Wilmut et al. 2006). When the gaze is off the reach target, other sensory signals such as arm proprioception exert a more prominent influence (Rossetti et al. 1995), which are likely to recruit different neural populations. Visually guided reaching thus involves ongoing comparison of different sensory inputs (e.g., visual vs. proprioceptive) to achieve maximum reach endpoint accuracy. Foveating the target could lead to longer movement times because of on-line corrective processes that combine ocular and retinal signals (Bédard and Proteau 2004; Desmurget and Grafton 2000). It has also been suggested that neurons in parietal cortex use retinal error signal that reflect the difference between target and hand position (Magescas et al. 2009). These error signals could be represented by PPC neurons during the different reach task conditions.
Direct comparisons of spatial tuning between RVAR and EVAR conditions during the preparatory and reach epochs showed distinct populations of cells. Spatial tuning during preparation and initiation epochs differed between RVAR and EVAR conditions for about one half of all neurons (T×P neurons). Task-related spatial changes in representations of reach planning and execution have been described for other parts of the cortex (Jouffrais and Boussaoud 1999; Schwartz et al. 1988). For example, motor cortex neurons are strongly affected by the kinetics of the arm movements (Scott and Kalaska 1997; Scott et al. 1997). Because the movement times in this study differed substantially between RVAR and EVAR conditions, the trajectories were not identical between the two task conditions.
These contextual effects can also be discussed with respect to different coordinate frames. Under both task conditions, reach movements were made to the same target location relative to the body (e.g., head, shoulders, or hands) but to different locations relative to the eye. If hand-centered signals dominated the 7a and DP responses, the spatial tuning would be similar between RVAR and EVAR conditions. If neural responses during reaching were dominated by eye-centered signals, spatial tuning would differ because eye position relative to the reach target (i.e., hand position) varied between RVAR and EVAR tasks. This was the case for the majority of cells, suggesting that eye position modulated tuning of these cells during the preparation and initiation of the reach movement. The changes of spatial tuning over time (between epochs) suggest that the initial eye position and retinotopic visual signals are affected later during the task by other reach-related signals. It is also likely that 7a and DP neurons use mixed coordinate frames, for example, a combination of hand-centered and eye-centered coordinates, as suggested for premotor and PRR neurons (Batista et al. 2007; Chang and Snyder 2010).
Reach-related activity in dorsal prelunate and area 7a neurons
Neurons in area 7a are strongly visual but show substantial modulation from extraretinal sources such as eye position (Andersen et al. 1990b; Read and Siegel 1997), attention (Constantinidis and Steinmetz 2001; Mountcastle et al. 1981; Quraishi et al. 2007; Raffi and Siegel 2005; Steinmetz and Constantinidis 1995; Steinmetz et al. 1994), and visually guided, goal-directed hand movements (Battaglia-Mayer et al. 2007; MacKay 1992; Rozzi et al. 2008; Rushworth et al. 1997).
DP neurons also showed modulation during preparation and initiation of reach movements, which was an unexpected result of this study. Properties of DP neurons strongly resembled those of area 7a with respect to temporal (epoch-based) and contextual (task-related) effects. Thus these results support a role for DP neurons that goes beyond the merely visual (Fischer and Boch 1981a; Mountcastle et al. 1987; Tanaka et al. 1986). Although DP is lower in an anatomically defined hierarchy than area 7a (Andersen et al. 1990a; Felleman and Van Essen 1991), it receives modulatory feedback from other parietal and frontal areas (Lewis and Van Essen 2000). The possibility that DP, and perhaps 7a, receive these signals via reentry (Edelman 1989) suggests a powerful role of these processes.
GRANTS
This work was supproted by National Institutes of Health Grants EY-09223 and 1S10 RR-12873, Whitehall Foundation, a Charles and Johanna Busch Faculty Research grant, and the National Partnership for Advanced Computational Infrastructure.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank H. Poizner and M. Raffi for contributions and J. Siegel and R. Meltzer for technical assistance.
REFERENCES
- Admiraal et al., 2004. Admiraal MA, Keijsers NLW, Gielen CCAM. Gaze affects pointing toward remembered visual targets after a self-initiated step. J Neurophysiol 92: 2380–2393, 2004 [DOI] [PubMed] [Google Scholar]
- Andersen, 1997. Andersen RA. Multimodal integration for the representation of space in the posterior parietal cortex. Philos Trans R Soc Lond B Biol Sci 352: 1421–1428, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen et al., 1990a. Andersen RA, Asanuma C, Essick G, Siegel RM. Corticocortical connections of anatomically and physiologically defined subdivisions within the inferior parietal lobule. J Comp Neurol 296: 65–113, 1990a [DOI] [PubMed] [Google Scholar]
- Andersen et al., 1990b. Andersen RA, Bracewell RM, Barash S, Gnadt JW, Fogassi L. Eye position effects on visual, memory, and saccade-related activity in areas LIP and 7a of macaque. J Neurosci 10: 1176–1196, 1990b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen et al., 1985. Andersen RA, Essick GK, Siegel RM. Encoding of spatial location by posterior parietal neurons. Science 230: 456–458, 1985 [DOI] [PubMed] [Google Scholar]
- Andersen and Mountcastle, 1983. Andersen RA, Mountcastle VB. The influence of the angle of gaze upon the excitability of the light- sensitive neurons of the posterior parietal cortex. J Neurosci 3: 532–548, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen et al., 1997. Andersen RA, Snyder LH, Bradley DC, Xing J. Multimodal representation of space in the posterior parietal cortex and its use in planning movements. Annu Rev Neurosci 20: 303–330, 1997 [DOI] [PubMed] [Google Scholar]
- Anderson and Siegel, 1999. Anderson KC, Siegel RM. Optic flow selectivity in the anterior superior temporal polysensory area, STPa, of the behaving monkey. J Neurosci 19: 2681–2692, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archambault et al., 2009. Archambault PS, Caminiti R, Battaglia-Mayer A. Cortical mechanisms for online control of hand movement trajectory: the role of the posterior parietal cortex. Cereb Cortex 19: 2848–2864, 2009 [DOI] [PubMed] [Google Scholar]
- Baraduc et al., 2001. Baraduc P, Guigon E, Burnod Y. Recoding arm position to learn visuomotor transformations. Cereb Cortex 11: 906–917, 2001 [DOI] [PubMed] [Google Scholar]
- Batista and Andersen, 2001. Batista AP, Andersen RA. The parietal reach region codes the next planned movement in a sequential reach task. J Neurophysiol 85: 539–544, 2001 [DOI] [PubMed] [Google Scholar]
- Batista et al., 1999. Batista AP, Buneo CA, Snyder LH, Andersen RA. Reach plans in eye-centered coordinates. Science 285: 257–260, 1999 [DOI] [PubMed] [Google Scholar]
- Batista et al., 2007. Batista AP, Santhanam G, Yu BM, Ryu SI, Afshar A, Shenoy KV. Reference frames for reach planning in macaque dorsal premotor cortex. J Neurophysiol 98: 966–983, 2007 [DOI] [PubMed] [Google Scholar]
- Batschelet, 1981. Batschelet E. Circular Statistics in Biology. London: Academic Press, 1981 [Google Scholar]
- Battaglia-Mayer et al., 2001. Battaglia-Mayer A, Ferraina S, Genovesio A, Marconi B, Squatrito S, Molinari M, Lacquaniti F, Caminiti R. Eye-hand coordination during reaching. II. An analysis of the relationships between visuomanual signals in parietal cortex and parieto-frontal association projections. Cereb Cortex 11: 528–544, 2001 [DOI] [PubMed] [Google Scholar]
- Battaglia-Mayer et al., 2005. Battaglia-Mayer A, Mascaro M, Brunamonti E, Caminiti R. The over-representation of contralateral space in parietal cortex: a positive image of directional motor components of neglect? Cereb Cortex 15: 514–525, 2005 [DOI] [PubMed] [Google Scholar]
- Battaglia-Mayer et al., 2007. Battaglia-Mayer A, Mascaro M, Caminiti R. Temporal evolution and strength of neural activity in parietal cortex during eye and hand movements. Cereb Cortex 17: 1350–1363, 2007 [DOI] [PubMed] [Google Scholar]
- Bédard and Proteau, 2004. Bédard P, Proteau L. On-line vs. off-line utilization of peripheral visual afferent information to ensure spatial accuracy of goal-directed movements. Exp Brain Res 158: 75–85, 2004 [DOI] [PubMed] [Google Scholar]
- Bender and Youakim, 2001. Bender DB, Youakim M. Effect of attentive fixation in macaque thalamus and cortex. J Neurophysiol 85: 219–234, 2001 [DOI] [PubMed] [Google Scholar]
- Beurze et al., 2007. Beurze SM, de Lange FP, Toni I, Medendorp WP. Integration of target and effector information in the human brain during reach planning. J Neurophysiol 97: 188–199, 2007 [DOI] [PubMed] [Google Scholar]
- Blum, 1985. Blum B. Manipulation reach and visual reach neurons in the inferior parietal lobule of the rhesus monkey. Behav Brain Res 18: 167–173, 1985 [DOI] [PubMed] [Google Scholar]
- Bremmer et al., 1997. Bremmer F, Distler C, Hoffmann KP. Eye position effects in monkey cortex. II. Pursuit- and fixation-related activity in posterior parietal areas LIP and 7A. J Neurophysiol 77: 962–977, 1997 [DOI] [PubMed] [Google Scholar]
- Breveglieri et al., 2006. Breveglieri R, Galletti C, Gamberini M, Passarelli L, Fattori P. Somatosensory cells in area PEc of macaque posterior parietal cortex. J Neurosci 26: 3679–3684, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breveglieri et al., 2008. Breveglieri R, Galletti C, Monaco S, Fattori P. Visual, somatosensory, and bimodal activities in the macaque parietal area PEc. Cereb Cortex 18: 806–816, 2008 [DOI] [PubMed] [Google Scholar]
- Bullock et al., 1998. Bullock D, Cisek P, Grossberg S. Cortical networks for control of voluntary arm movements under variable force conditions. Cereb Cortex 8: 48–62, 1998 [DOI] [PubMed] [Google Scholar]
- Buneo et al., 2002. Buneo CA, Jarvis MR, Batista AP, Andersen RA. Direct visuomotor transformations for reaching. Nature 416: 632–636, 2002 [DOI] [PubMed] [Google Scholar]
- Burnod et al., 1999. Burnod Y, Baraduc P, Battaglia-Mayer A, Guigon E, Koechlin E, Ferraina S, Lacquaniti F, Caminiti R. Parieto-frontal coding of reaching: an integrated framework. Exp Brain Res 129: 325–346, 1999 [DOI] [PubMed] [Google Scholar]
- Bushnell et al., 1981. Bushnell MC, Goldberg ME, Robinson DL. Behavioral enhancement of visual responses in monkey cerebral cortex. I. Modulation in posterior parietal cortex related to selective visual attention. J Neurophysiol 46: 755–772, 1981 [DOI] [PubMed] [Google Scholar]
- Buxbaum and Coslett, 1998. Buxbaum LJ, Coslett H. Spatio-motor representations in reaching: evidence for subtypes of optic ataxia. Cogn Neuropsychol 15: 279–312, 1998 [DOI] [PubMed] [Google Scholar]
- Calton et al., 2002. Calton JL, Dickinson AR, Snyder LH. Non-spatial, motor-specific activation in posterior parietal cortex. Nat Neurosci 5: 580–588, 2002 [DOI] [PubMed] [Google Scholar]
- Cavada and Goldman-Rakic, 1989a. Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus monkey. I. Parcellation of areas based on distinctive limbic and sensory corticocortical connections. J Comp Neurol 287: 393–421, 1989a [DOI] [PubMed] [Google Scholar]
- Cavada and Goldman-Rakic, 1989b. Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus monkey. II. Evidence for segregated corticocortical networks linking sensory and limbic areas with the frontal lobe. J Comp Neurol 287: 422–445, 1989b [DOI] [PubMed] [Google Scholar]
- Chang and Snyder, 2010. Chang SWC, Snyder LH. Idiosyncratic and systematic aspects of spatial representations in the macaque parietal cortex. Proc Natl Acad Sci USA 107: 7951–7956, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen et al., 2009. Chen J, Reitzen SD, Kohlenstein JB, Gardner EP. Neural representation of hand kinematics during prehension in posterior parietal cortex of the macaque monkey. J Neurophysiol 102: 3310–3328, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchland et al., 2006. Churchland MM, Santhanam G, Shenoy KV. Preparatory activity in premotor and motor cortex reflects the speed of the upcoming reach. J Neurophysiol 96: 3130–3146, 2006 [DOI] [PubMed] [Google Scholar]
- Clavagnier et al., 2007. Clavagnier S, Prado J, Kennedy H, Perenin M-T. How humans reach: distinct cortical systems for central and peripheral vision. Neuroscientist 13: 22–27, 2007 [DOI] [PubMed] [Google Scholar]
- Constantinidis and Steinmetz, 2001. Constantinidis C, Steinmetz MA. Neuronal responses in area 7a to multiple-stimulus displays. I. Neurons encode the location of the salient stimulus. Cereb Cortex 11: 581–591, 2001 [DOI] [PubMed] [Google Scholar]
- Coulthard et al., 2008. Coulthard EJ, Nachev P, Husain M. Control over conflict during movement preparation: role of posterior parietal cortex. Neuron 58: 144–157, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmurget et al., 1999. Desmurget M, Epstein CM, Turner RS, Prablanc C, Alexander GE, Grafton ST. Role of the posterior parietal cortex in updating reaching movements to a visual target. Nat Neurosci 2: 563–567, 1999 [DOI] [PubMed] [Google Scholar]
- Desmurget and Grafton, 2000. Desmurget M, Grafton S. Forward modeling allows feedback control for fast reaching movements. Trends Cogn Sci 4: 423–431, 2000 [DOI] [PubMed] [Google Scholar]
- Desmurget et al., 1998. Desmurget M, Pelisson D, Rossetti Y, Prablanc C. From eye to hand: planning goal-directed movements. Neurosci Biobehav Rev 22: 761–788, 1998 [DOI] [PubMed] [Google Scholar]
- Dijkerman et al., 2006. Dijkerman HC, McIntosh RD, Anema HA, de Haan EHF, Kappelle LJ, Milner AD. Reaching errors in optic ataxia are linked to eye position rather than head or body position. Neuropsychologia 44: 2766–2773, 2006 [DOI] [PubMed] [Google Scholar]
- Edelman, 1989. Edelman GM. The Remembered Present: A Biological Theory of Consciousness. New York: Basics Books, 1989 [Google Scholar]
- Fattori et al., 2001. Fattori P, Gamberini M, Kutz DF, Galletti C. ‘Arm-reaching’ neurons in the parietal area V6A of the macaque monkey. Eur J Neurosci 13: 2309–2313, 2001 [DOI] [PubMed] [Google Scholar]
- Fattori et al., 2005. Fattori P, Kutz DF, Breveglieri R, Marzocchi N, Galletti C. Spatial tuning of reaching activity in the medial parieto-occipital cortex (area V6A) of macaque monkey. Eur J Neurosci 22: 956–972, 2005 [DOI] [PubMed] [Google Scholar]
- Felleman and Van Essen, 1991. Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex 1: 1–47, 1991 [DOI] [PubMed] [Google Scholar]
- Ferraina et al., 2001. Ferraina S, Battaglia-Mayer A, Genovesio A, Marconi B, Onorati P, Caminiti R. Early coding of visuomanual coordination during reaching in parietal area PEc. J Neurophysiol 85: 462–467, 2001 [DOI] [PubMed] [Google Scholar]
- Ferraina et al., 1997. Ferraina S, Garasto MR, Battaglia-Mayer A, Ferraresi P, Johnson PB, Lacquaniti F, Carniniti R. Visual control of hand-reaching movement: activity in parietal area 7m. Eur J Neurosci 9: 1090–1095, 1997 [DOI] [PubMed] [Google Scholar]
- Fischer and Boch, 1981a. Fischer B, Boch R. Enhanced activation of neurons in prelunate cortex before visually guided saccades of trained rhesus monkeys. Exp Brain Res 44: 129–137, 1981a [DOI] [PubMed] [Google Scholar]
- Fischer and Boch, 1981b. Fischer B, Boch R. Selection of visual targets activates prelunate cortical cells in trained rhesus monkey. Exp Brain Res 41: 431–433, 1981b [DOI] [PubMed] [Google Scholar]
- Gardner et al., 2007. Gardner EP, Babu KS, Reitzen SD, Ghosh S, Brown AS, Chen J, Hall AL, Herzlinger MD, Kohlenstein JB, Ro JY. Neurophysiology of prehension. I. Posterior parietal cortex and object-oriented hand behaviors. J Neurophysiol 97: 387–406, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghika et al., 1995. Ghika J, Bogousslavsky J, Uske A, Regli F. Parietal kinetic ataxia without proprioceptive deficit. J Neurol Neurosurg Psychiatry 59: 531–533, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg et al., 2002. Goldberg ME, Bisley J, Powell KD, Gottlieb J, Kusunoki M. The role of the lateral intraparietal area of the monkey in the generation of saccades and visuospatial attention. Ann NY Acad Sci 956: 205–215, 2002 [DOI] [PubMed] [Google Scholar]
- Heider et al., 2005. Heider B, Jando G, Siegel RM. Functional architecture of retinotopy in visual association cortex of behaving monkey. Cereb Cortex 15: 460–478, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriques and Crawford, 2000. Henriques DYP, Crawford JD. Direction-dependent distortions of retinocentric space in the visuomotor transformation for pointing. Exp Brain Res 132: 179–194, 2000 [DOI] [PubMed] [Google Scholar]
- Henriques et al., 1998. Henriques DYP, Klier EM, Smith MA, Lowy D, Crawford JD. Gaze-centered remapping of remembered visual space in an open-loop pointing task. J Neurosci 18: 1583–1594, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyvärinen, 1982. Hyvärinen J. The Parietal Cortex of Monkey and Man. Berlin: Springer-Verlag, 1982 [Google Scholar]
- Johnson et al., 1996. Johnson PB, Ferraina S, Bianchi L, Caminiti R. Cortical networks for visual reaching: physiological and anatomical organization of frontal and parietal lobe arm regions. Cereb Cortex 6: 102–119, 1996 [DOI] [PubMed] [Google Scholar]
- Jouffrais and Boussaoud, 1999. Jouffrais C, Boussaoud D. Neuronal activity related to eye-hand coordination in the primate premotor cortex. Exp Brain Res 128: 205–209, 1999 [DOI] [PubMed] [Google Scholar]
- Kalaska, 1988. Kalaska JF. The representation of arm movements in postcentral and parietal cortex. Can J Physiol Pharmacol 66: 455–463, 1988 [DOI] [PubMed] [Google Scholar]
- Kurata and Hoshi, 2002. Kurata K, Hoshi E. Movement-related neuronal activity reflecting the transformation of coordinates in the ventral premotor cortex of monkeys. J Neurophysiol 88: 3118–3132, 2002 [DOI] [PubMed] [Google Scholar]
- Lewis and Van Essen, 2000. Lewis JW, Van Essen DC. Corticocortical connections of visual, sensorimotor, and multimodal processing areas in the parietal lobe of the macaque monkey. J Comp Neurol 428: 112–137, 2000 [DOI] [PubMed] [Google Scholar]
- Lewis et al., 1998. Lewis RF, Gaymard BM, Tamargo RJ. Efference copy provides the eye position information required for visually guided reaching. J Neurophysiol 80: 1605–1608, 1998 [DOI] [PubMed] [Google Scholar]
- Liu et al., 2010. Liu Y, Yttri EA, Snyder LH. Intention and attention: different functional roles for LIPd and LIPv. Nat Neurosci 13: 495–500, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKay, 1992. MacKay WA. Properties of reach-related neuronal activity in cortical area 7A. J Neurophysiol 67: 1335–1345, 1992 [DOI] [PubMed] [Google Scholar]
- Magescas et al., 2009. Magescas F, Urquizar C, Prablanc C. Two modes of error processing in reaching. Exp Brain Res 193: 337–350, 2009 [DOI] [PubMed] [Google Scholar]
- Maguire and Baizer, 1984. Maguire WM, Baizer JS. Visuotopic organization of the prelunate gyrus in rhesus monkey. J Neurosci 4: 1690–1704, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzocchi et al., 2008. Marzocchi N, Breveglieri R, Galletti C, Fattori P. Reaching activity in parietal area V6A of macaque: eye influence on arm activity or retinocentric coding of reaching movements?. Eur J Neurosci 27: 775–789, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant et al., 2001. Merchant H, Battaglia-Mayer A, Georgopoulos AP. Effects of optic flow in motor cortex and area 7a. J Neurophysiol 86: 1937–1954, 2001 [DOI] [PubMed] [Google Scholar]
- Moran and Schwartz, 1999. Moran DW, Schwartz AB. Motor cortical representation of speed and direction during reaching. J Neurophysiol 82: 2676–2692, 1999 [DOI] [PubMed] [Google Scholar]
- Motter and Mountcastle, 1981. Motter BC, Mountcastle VB. The functional properties of the light-sensitive neurons of the posterior parietal cortex studied in waking monkeys: foveal sparing and opponent vector organization. J Neurosci 1: 3–26, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountcastle et al., 1981. Mountcastle VB, Andersen RA, Motter BC. The influence of attentive fixation upon the excitability of the light-sensitive neurons of the posterior parietal cortex. J Neurosci 1: 1218–1225, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountcastle et al., 1975. Mountcastle VB, Lynch JC, Georgopoulos A, Sakata H, Acuna C. Posterior parietal association cortex of the monkey: command functions for operations within extrapersonal space. J Neurophysiol 38: 871–908, 1975 [DOI] [PubMed] [Google Scholar]
- Mountcastle et al., 1987. Mountcastle VB, Motter BC, Steinmetz MA, Sestokas AK. Common and differential effects of attentive fixation on the excitability of parietal and prestriate (V4) cortical visual neurons in the macaque monkey. J Neurosci 7: 2239–2255, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neggers and Bekkering, 2001. Neggers SFW, Bekkering H. Gaze anchoring to a pointing target is present during the entire pointing movement and is driven by a non-visual signal. J Neurophysiol 86: 961–970, 2001 [DOI] [PubMed] [Google Scholar]
- Pesaran et al., 2006. Pesaran B, Nelson MJ, Andersen RA. Dorsal premotor neurons encode the relative position of the hand, eye, and goal during reach planning. Neuron 51: 125–134, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisella et al., 2000. Pisella L, Grea H, Tilikete C, Vighetto A, Desmurget M, Rode G, Boisson D, Rossetti Y. An ‘automatic pilot’ for the hand in human posterior parietal cortex: toward reinterpreting optic ataxia. Nat Neurosci 3: 729–736, 2000 [DOI] [PubMed] [Google Scholar]
- Pisella et al., 2009. Pisella L, Sergio L, Blangero A, Torchin H, Vighetto A, Rossetti Y. Optic ataxia and the function of the dorsal stream: contributions to perception and action. Neuropsychologia 47: 3033–3044, 2009 [DOI] [PubMed] [Google Scholar]
- Prablanc et al., 1986. Prablanc C, Pélisson D, Goodale MA. Visual control of reaching movements without vision of the limb. Exp Brain Res 62: 293–302, 1986 [DOI] [PubMed] [Google Scholar]
- Prud'homme and Kalaska, 1994. Prud'homme MJ, Kalaska JF. Proprioceptive activity in primate primary somatosensory cortex during active arm reaching movements. J Neurophysiol 72: 2280–2301, 1994 [DOI] [PubMed] [Google Scholar]
- Quraishi et al., 2007. Quraishi S, Heider B, Siegel RM. Attentional modulation of receptive field structure in area 7a of the behaving monkey. Cereb Cortex 17: 1841–1857, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffi and Siegel, 2005. Raffi M, Siegel RM. Functional architecture of spatial attention in the parietal cortex of the behaving monkey. J Neurosci 25: 5171–5186, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read and Siegel, 1997. Read HL, Siegel RM. Modulation of responses to optic flow in area 7a by retinotopic and oculomotor cues in monkey. Cereb Cortex 7: 647–661, 1997 [DOI] [PubMed] [Google Scholar]
- Ren et al., 2006. Ren L, Khan AZ, Blohm G, Henriques DYP, Sergio LE, Crawford JD. Proprioceptive guidance of saccades in eye-hand coordination. J Neurophysiol 96: 1464–1477, 2006 [DOI] [PubMed] [Google Scholar]
- Rossetti et al., 1995. Rossetti Y, Desmurget M, Prablanc C. Vectorial coding of movement: vision, proprioception, or both? J Neurophysiol 74: 457–463, 1995 [DOI] [PubMed] [Google Scholar]
- Rozzi et al., 2006. Rozzi S, Calzavara R, Belmalih A, Borra E, Gregoriou GG, Matelli M, Luppino G. Cortical connections of the inferior parietal cortical convexity of the macaque monkey. Cereb Cortex 16: 1389–1417, 2006 [DOI] [PubMed] [Google Scholar]
- Rozzi et al., 2008. Rozzi S, Ferrari PF, Bonini L, Rizzolatti G, Fogassi L. Functional organization of inferior parietal lobule convexity in the macaque monkey: electrophysiological characterization of motor, sensory and mirror responses and their correlation with cytoarchitectonic areas. Eur J Neurosci 28: 1569–1588, 2008 [DOI] [PubMed] [Google Scholar]
- Rushworth et al., 1997. Rushworth MF, Nixon PD, Passingham RE. Parietal cortex and movement. I. Movement selection and reaching. Exp Brain Res 117: 292–310, 1997 [DOI] [PubMed] [Google Scholar]
- Sakata et al., 1995. Sakata H, Taira M, Murata A, Mine S. Neural mechanisms of visual guidance of hand action in the parietal cortex of the monkey. Cereb Cortex 5: 429–438, 1995 [DOI] [PubMed] [Google Scholar]
- Salinas and Sejnowski, 2001. Salinas E, Sejnowski TJ. Book review: gain modulation in the central nervous system: where behavior, neurophysiology, and computation meet. Neuroscientist 7: 430–440, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidt et al., 2005. Scheidt RA, Conditt MA, Secco EL, Mussa-Ivaldi FA. Interaction of visual and proprioceptive feedback during adaptation of human reaching movements. J Neurophysiol 93: 3200–3213, 2005 [DOI] [PubMed] [Google Scholar]
- Scherberger and Andersen, 2007. Scherberger H, Andersen RA. Target selection signals for arm reaching in the posterior parietal cortex. J Neurosci 27: 2001–2012, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherberger et al., 2003. Scherberger H, Goodale MA, Andersen RA. Target selection for reaching and saccades share a similar behavioral reference frame in the macaque. J Neurophysiol 89: 1456–1466, 2003 [DOI] [PubMed] [Google Scholar]
- Scherberger et al., 2005. Scherberger H, Jarvis MR, Andersen RA. Cortical local field potential encodes movement intentions in the posterior parietal cortex. Neuron 46: 347–354, 2005 [DOI] [PubMed] [Google Scholar]
- Schwartz et al., 1988. Schwartz A, Kettner R, Georgopoulos A. Primate motor cortex and free arm movements to visual targets in three- dimensional space. I. Relations between single cell discharge and direction of movement. J Neurosci 8: 2913–2927, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott and Kalaska, 1997. Scott SH, Kalaska JF. Reaching movements with similar hand paths but different arm orientations. I. Activity of individual cells in motor cortex. J Neurophysiol 77: 826–852, 1997 [DOI] [PubMed] [Google Scholar]
- Scott et al., 1997. Scott SH, Sergio LE, Kalaska JF. Reaching movements with similar hand paths but different arm orientations. II. Activity of individual cells in dorsal premotor cortex and parietal area 5. J Neurophysiol 78: 2413–2426, 1997 [DOI] [PubMed] [Google Scholar]
- Shadmehr and Wise, 2005. Shadmehr R, Wise SP. The Computational Neurobiology of Reaching and Pointing: A Foundation for Motor Learning. Cambridge, MA: MIT Press, 2005 [Google Scholar]
- Siegel et al., 2007. Siegel RM, Duann JR, Jung TP, Sejnowski T. Spatiotemporal dynamics of the functional architecture for gain fields in inferior parietal lobule of behaving monkey. Cereb Cortex 17: 378–390, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel et al., 2003. Siegel RM, Raffi M, Phinney RE, Turner JA, Jando G. Functional architecture of eye position gain fields in visual association cortex of behaving monkey. J Neurophysiol 90: 1279–1294, 2003 [DOI] [PubMed] [Google Scholar]
- Siegel and Read, 1997. Siegel RM, Read HL. Analysis of optic flow in the monkey parietal area 7a. Cereb Cortex 7: 327–346, 1997 [DOI] [PubMed] [Google Scholar]
- Snyder et al., 1997. Snyder LH, Batista AP, Andersen RA. Coding of intention in the posterior parietal cortex. Nature 386: 167–170, 1997 [DOI] [PubMed] [Google Scholar]
- Snyder et al., 1998. Snyder LH, Batista AP, Andersen RA. Change in motor plan, without a change in the spatial locus of attention, modulates activity in posterior parietal cortex. J Neurophysiol 79: 2814–2819, 1998 [DOI] [PubMed] [Google Scholar]
- Snyder et al., 2000. Snyder LH, Batista AP, Andersen RA. Intention-related activity in the posterior parietal cortex: a review. Vision Res 40: 1433–1441, 2000 [DOI] [PubMed] [Google Scholar]
- Snyder et al., 2006. Snyder LH, Dickinson AR, Calton JL. Preparatory delay activity in the monkey parietal reach region predicts reach reaction times. J Neurosci 26: 10091–10099, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz et al., 1994. Steinmetz MA, Connor CE, Constantinidis C, McLaughlin JR. Covert attention suppresses neuronal responses in area 7a of the posterior parietal cortex. J Neurophysiol 72: 1020–1023, 1994 [DOI] [PubMed] [Google Scholar]
- Steinmetz and Constantinidis, 1995. Steinmetz MA, Constantinidis C. Neurophysiological evidence for a role of posterior parietal cortex in redirecting visual attention. Cereb Cortex 5: 448–456, 1995 [DOI] [PubMed] [Google Scholar]
- Stepniewska et al., 2005. Stepniewska I, Collins CE, Kaas JH. Reappraisal of DL/V4 boundaries based on connectivity patterns of dorsolateral visual cortex in macaques. Cereb Cortex 15: 809–822, 2005 [DOI] [PubMed] [Google Scholar]
- Tanaka et al., 1986. Tanaka M, Weber H, Creutzfeldt OD. Visual properties and spatial distribution of neurones in the visual association area on the prelunate gyrus of the awake monkey. Exp Brain Res 65: 11–37, 1986 [DOI] [PubMed] [Google Scholar]
- Vercher et al., 1994. Vercher JL, Magenes G, Prablanc C, Gauthier GM. Eye-head-hand coordination in pointing at visual targets: spatial and temporal analysis. Exp Brain Res 99: 507–523, 1994 [DOI] [PubMed] [Google Scholar]
- Wilmut et al., 2006. Wilmut K, Wann J, Brown J. How active gaze informs the hand in sequential pointing movements. Exp Brain Res 175: 654–666, 2006 [DOI] [PubMed] [Google Scholar]
- Youakim et al., 2001. Youakim M, Bender DB, Baizer JS. Vertical meridian representation on the prelunate gyrus in area V4 of macaque. Brain Res Bull 56: 93–100, 2001 [DOI] [PubMed] [Google Scholar]
- Zar, 1984. Zar JH. Biostatistical Analysis. Englewood Cliffs, NJ: Prentice-Hall, 1984 [Google Scholar]