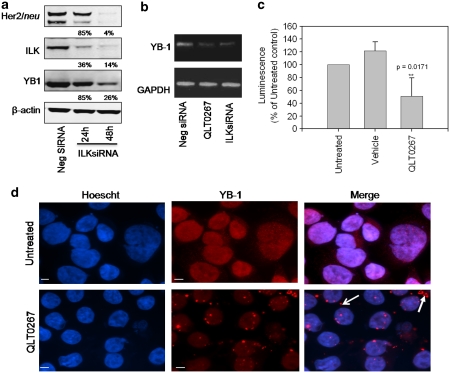
Figure 3.
Inhibition of ILK activity or expression influences YB-1 transcription and subcellular localization. (a) SKBR3 cells were transiently nucleofected with 4 μg ILK siRNA. Subsequently, cells were lysed and 50 μg of protein was isolated from samples at 24 and 48 h, separated on a 10% SDS–PAGE gel and probed for ILK, Her2/neu, YB-1 and β-actin to verify loading. ILK expression was substantially silenced when SKBR3 cells were treated with 4 μg of ILK siRNA for both 24 and 48 h. Cells exhibit a 96% decrease in total Her2/neu expression after 48 h, at which time YB-1 expression is reduced by 74%. (b) YB-1 transcript levels were analyzed in SKBR3 cells treated with QLT0267 or nucleofected with 4 μg ILK siRNA for 48 h using PCR. A 9.9-fold and 6.5-fold decrease in YB-1 transcript was observed in QLT0267-treated and ILK-silenced cells, respectively, when compared with control. (c) SKBR3 cells were transfected with a YB-1 promoter/luciferase construct and treated with QLT0267 or vehicle control (PTE) for 24 h. A significant reduction in YB-1 promoter activity of 50% is achieved when cells are treated with QLT0267 when compared with untreated controls (P<0.05) (d) SKBR3 cells grown on coverslips were treated with 42 μ QLT0267 for 24 h, fixed with 4% paraformaldehyde (PFA) and then stained for YB-1. Immunofluorescent images show that treatment of SKBR3 cells trigger a decrease in YB-1 protein (red) as well as a change in localization to granular structures in the cytoplasm (white arrows). Hoechst staining was used to counter stain nuclei (blue). Bar, 5 μm.