Abstract
In the title compound, C6H9N2 +·C7H5O3 −, the protonated 2-amino-5-methylpyridinium cation and the 2-hydroxybenzoate anion are both essentially planar, with maximum deviations of 0.026 (2) and 0.034 (1) Å, respectively. The anion is stabilized by an intramolecular O—H⋯O hydrogen bond, which forms an S(6) ring motif. In the solid state, the anions are linked to the cations via pairs of intermolecular N—H⋯O hydrogen bonds forming R 2 2(8) ring motifs. The crystal structure is further stabilized by N—H⋯O and C—H⋯O interactions which link the molecules into chains along [010]. A π–π stacking interaction [centroid–centroid-distance = 3.740 (2) Å] is also observed.
Related literature
For background to and the applications of carboxylic acids, see: Miller & Orgel (1974 ▶); Kvenvolden et al. (1971 ▶); Desiraju (1989 ▶); MacDonald & Whitesides (1994 ▶). For applications of salicylic acid, see: Singh & Vijayan (1974 ▶); Patel et al. (1988 ▶). For related structures, see: Quah et al. (2008 ▶; 2010a
▶,b
▶). For bond-length data, see: Allen et al. (1987 ▶). For hydrogen-bond motifs, see: Bernstein et al. (1995 ▶).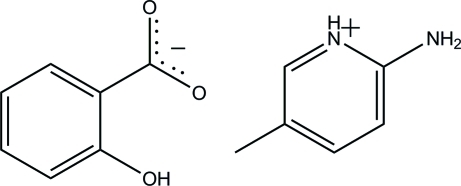
Experimental
Crystal data
C6H9N2 +·C7H5O3 −
M r = 246.26
Monoclinic,

a = 13.211 (7) Å
b = 7.170 (4) Å
c = 14.324 (7) Å
β = 104.668 (11)°
V = 1312.6 (12) Å3
Z = 4
Mo Kα radiation
μ = 0.09 mm−1
T = 297 K
0.42 × 0.19 × 0.10 mm
Data collection
Bruker SMART APEXII DUO CCD area-detector diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 2009 ▶) T min = 0.963, T max = 0.991
14312 measured reflections
3797 independent reflections
2233 reflections with I > 2σ(I)
R int = 0.028
Refinement
R[F 2 > 2σ(F 2)] = 0.043
wR(F 2) = 0.134
S = 1.01
3797 reflections
219 parameters
All H-atom parameters refined
Δρmax = 0.14 e Å−3
Δρmin = −0.15 e Å−3
Data collection: APEX2 (Bruker, 2009 ▶); cell refinement: SAINT (Bruker, 2009 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXTL (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXTL; molecular graphics: SHELXTL; software used to prepare material for publication: SHELXTL and PLATON (Spek, 2009 ▶).
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536810030928/bt5314sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810030928/bt5314Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N1—H1N1⋯O3 | 1.03 (2) | 1.65 (2) | 2.678 (2) | 174.6 (13) |
| N2—H1N2⋯O2i | 0.884 (18) | 1.987 (17) | 2.852 (2) | 165.4 (14) |
| N2—H2N2⋯O2 | 0.97 (2) | 1.90 (2) | 2.872 (2) | 179 (2) |
| O1—H1O1⋯O3 | 1.03 (2) | 1.55 (2) | 2.515 (2) | 155 (2) |
| C5—H5A⋯O1ii | 0.961 (14) | 2.598 (14) | 3.518 (3) | 160.2 (10) |
Symmetry codes: (i)  ; (ii)
; (ii)  .
.
Acknowledgments
The authors thank Universiti Sains Malaysia (USM) for the Research University Golden Goose Grant (1001/PFIZIK/811012). CKQ also thanks USM for the award of USM fellowship and HM also thanks USM for the award of post doctoral fellowship.
supplementary crystallographic information
Comment
Hydrogen bonding has been established as the most effective tool for constructing sophisticated assemblies because of its strength and directionality. Carboxylic acids are believed to have existed in the prebiotic earth (Miller & Orgel, 1974; Kvenvolden et al., 1971) and they exhibit characteristic intermolecular interactions and aggregation patterns. Also carboxyl groups have been used as primary building blocks in the design of crystal structures (Desiraju, 1989; MacDonald & Whitesides, 1994). Salicylic acid, a well known analgesic, and its complexes with a few drug molecules such as antipyrine (Singh & Vijayan, 1974) and sulfadimidine (Patel et al., 1988) were already reported in the literature. The present study is aimed at investigating the supramolecular interactions of the title compound, (I).
The asymmetric unit of title compound (Fig. 1), contains a protonated 2-amino-5-methylpyridinium cation and a 2-hydroxybenzoate anion. In the 2-amino-5-methylpyridinium cation, a wide angle [122.26 (13)°] is subtended at the protonated N1 atom. The 2-amino-5-methylpyridinium cation and 2-hydroxybenzoate anion are essentially planar, with a maximum deviation of 0.026 (2) Å for atom C6 and 0.034 (1) Å for atom O3, respectively. The diheral angle between these two planes is 4.78 (5)°, indicating they are nearly parallel to each other. The anion is stabilized by an intramolecular O1–H1O1···O3 hydrogen bond, which forms an S(6) ring motif (Bernstein et al., 1995).
In the solid state (Fig. 2), the anions are linked to the cations via intermolecular N1–H1N1···O3 and N2–H2N2..O2 hydrogen bonds forming R22(8) ring motifs. The crystal structure is further stabilized by N2–H1N2···O2 and C5–H5A···O1 interactions. The molecules are linked by these interactions into chains along [010]. π–π stacking interactions with short intermolecular distance [3.740 (2) Å] between symmetry-related N1/C1—C5 (centroid Cg1) and C7—C12 (centroid Cg2) [symmetry code: x, 1 + y, z] are also observed.
Experimental
A hot methanol solution (20 ml) of 2-amino-5-methylpyridine (54 mg, Aldrich) and salicylic acid (34.5 mg, Merck) was mixed and warmed over a magnetic stirrer hotplate for a few minutes. The resulting solution was allowed to cool slowly at room temperature and crystals of the title compound appeared after a few days.
Refinement
All H atoms were located in a difference Fourier map and refined freely.
Figures
Fig. 1.
The molecular structure of the title compound showing 50% probability displacement ellipsoids for non-H atoms and the atom-numbering scheme. Intramolecular interactions are shown in dashed lines.
Fig. 2.
The crystal structure of the title compound viewed along the c axis.
Crystal data
| C6H9N2+·C7H5O3− | F(000) = 520 |
| Mr = 246.26 | Dx = 1.246 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2ybc | Cell parameters from 3026 reflections |
| a = 13.211 (7) Å | θ = 3.2–26.8° |
| b = 7.170 (4) Å | µ = 0.09 mm−1 |
| c = 14.324 (7) Å | T = 297 K |
| β = 104.668 (11)° | Block, yellow |
| V = 1312.6 (12) Å3 | 0.42 × 0.19 × 0.10 mm |
| Z = 4 |
Data collection
| Bruker SMART APEXII DUO CCD area-detector diffractometer | 3797 independent reflections |
| Radiation source: fine-focus sealed tube | 2233 reflections with I > 2σ(I) |
| graphite | Rint = 0.028 |
| φ and ω scans | θmax = 30.0°, θmin = 2.9° |
| Absorption correction: multi-scan (SADABS; Bruker, 2009) | h = −18→18 |
| Tmin = 0.963, Tmax = 0.991 | k = −10→10 |
| 14312 measured reflections | l = −20→19 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.043 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.134 | All H-atom parameters refined |
| S = 1.01 | w = 1/[σ2(Fo2) + (0.0607P)2 + 0.0959P] where P = (Fo2 + 2Fc2)/3 |
| 3797 reflections | (Δ/σ)max < 0.001 |
| 219 parameters | Δρmax = 0.14 e Å−3 |
| 0 restraints | Δρmin = −0.15 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| N1 | 0.25056 (7) | 0.79968 (17) | 0.30963 (8) | 0.0491 (3) | |
| N2 | 0.07534 (9) | 0.7373 (2) | 0.28908 (10) | 0.0664 (4) | |
| C1 | 0.15649 (8) | 0.84904 (19) | 0.32288 (9) | 0.0485 (3) | |
| C2 | 0.15072 (10) | 1.0168 (2) | 0.37198 (9) | 0.0542 (3) | |
| C3 | 0.23752 (10) | 1.1242 (2) | 0.40264 (10) | 0.0561 (3) | |
| C4 | 0.33511 (10) | 1.0709 (2) | 0.38749 (9) | 0.0544 (3) | |
| C5 | 0.33759 (9) | 0.9075 (2) | 0.34103 (9) | 0.0523 (3) | |
| C6 | 0.43050 (14) | 1.1912 (3) | 0.41915 (16) | 0.0791 (5) | |
| O1 | 0.40974 (7) | 0.27586 (19) | 0.17167 (9) | 0.0806 (4) | |
| O2 | 0.10956 (7) | 0.41138 (14) | 0.18462 (8) | 0.0703 (3) | |
| O3 | 0.27788 (7) | 0.48394 (15) | 0.21915 (9) | 0.0710 (3) | |
| C7 | 0.32912 (10) | 0.1574 (2) | 0.13606 (10) | 0.0581 (4) | |
| C8 | 0.35051 (15) | −0.0109 (3) | 0.09641 (12) | 0.0760 (5) | |
| C9 | 0.27204 (17) | −0.1348 (3) | 0.05976 (13) | 0.0830 (5) | |
| C10 | 0.17009 (17) | −0.0959 (3) | 0.06012 (13) | 0.0799 (5) | |
| C11 | 0.14807 (12) | 0.0712 (2) | 0.09921 (11) | 0.0635 (4) | |
| C12 | 0.22624 (9) | 0.19952 (19) | 0.13827 (9) | 0.0494 (3) | |
| C13 | 0.20156 (9) | 0.37630 (19) | 0.18311 (10) | 0.0529 (3) | |
| H2A | 0.0850 (11) | 1.050 (2) | 0.3827 (10) | 0.067 (4)* | |
| H3A | 0.2348 (12) | 1.246 (3) | 0.4373 (11) | 0.074 (5)* | |
| H5A | 0.3970 (10) | 0.854 (2) | 0.3237 (9) | 0.053 (3)* | |
| H6A | 0.4887 (16) | 1.146 (3) | 0.3935 (14) | 0.107 (7)* | |
| H6B | 0.4150 (17) | 1.318 (4) | 0.3879 (17) | 0.131 (9)* | |
| H6C | 0.4491 (16) | 1.208 (3) | 0.4862 (19) | 0.123 (8)* | |
| H8A | 0.4216 (15) | −0.033 (3) | 0.1009 (13) | 0.097 (6)* | |
| H9A | 0.2895 (14) | −0.256 (3) | 0.0343 (13) | 0.098 (6)* | |
| H10A | 0.1156 (15) | −0.181 (3) | 0.0375 (14) | 0.102 (6)* | |
| H11A | 0.0773 (12) | 0.101 (2) | 0.1003 (10) | 0.068 (4)* | |
| H1N1 | 0.2564 (11) | 0.678 (3) | 0.2733 (11) | 0.073 (4)* | |
| H1N2 | 0.0141 (13) | 0.771 (2) | 0.2980 (12) | 0.078 (5)* | |
| H2N2 | 0.0862 (13) | 0.626 (3) | 0.2540 (13) | 0.087 (5)* | |
| H1O1 | 0.3730 (16) | 0.382 (3) | 0.1984 (15) | 0.116 (7)* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| N1 | 0.0397 (5) | 0.0494 (7) | 0.0589 (6) | 0.0009 (5) | 0.0137 (4) | −0.0009 (5) |
| N2 | 0.0401 (5) | 0.0620 (9) | 0.0988 (9) | −0.0039 (5) | 0.0209 (6) | −0.0157 (7) |
| C1 | 0.0396 (5) | 0.0504 (8) | 0.0560 (7) | 0.0017 (5) | 0.0131 (5) | 0.0018 (6) |
| C2 | 0.0480 (6) | 0.0565 (9) | 0.0592 (7) | 0.0039 (6) | 0.0158 (6) | −0.0028 (6) |
| C3 | 0.0614 (7) | 0.0537 (9) | 0.0520 (7) | −0.0011 (7) | 0.0121 (6) | −0.0041 (7) |
| C4 | 0.0524 (7) | 0.0579 (9) | 0.0508 (7) | −0.0090 (6) | 0.0092 (5) | 0.0044 (6) |
| C5 | 0.0401 (6) | 0.0607 (9) | 0.0574 (7) | −0.0017 (6) | 0.0143 (5) | 0.0045 (7) |
| C6 | 0.0653 (9) | 0.0833 (14) | 0.0848 (12) | −0.0264 (10) | 0.0118 (9) | −0.0071 (11) |
| O1 | 0.0495 (5) | 0.0882 (9) | 0.1105 (9) | 0.0064 (5) | 0.0323 (5) | −0.0146 (7) |
| O2 | 0.0473 (5) | 0.0544 (7) | 0.1169 (8) | 0.0028 (4) | 0.0349 (5) | −0.0095 (6) |
| O3 | 0.0502 (5) | 0.0530 (6) | 0.1159 (8) | −0.0031 (5) | 0.0324 (5) | −0.0166 (6) |
| C7 | 0.0549 (7) | 0.0635 (10) | 0.0597 (7) | 0.0154 (7) | 0.0216 (6) | 0.0049 (7) |
| C8 | 0.0784 (10) | 0.0775 (13) | 0.0775 (10) | 0.0292 (10) | 0.0297 (8) | −0.0028 (9) |
| C9 | 0.1128 (15) | 0.0655 (12) | 0.0739 (10) | 0.0248 (11) | 0.0296 (10) | −0.0121 (9) |
| C10 | 0.0967 (13) | 0.0645 (12) | 0.0772 (11) | −0.0022 (10) | 0.0198 (9) | −0.0197 (9) |
| C11 | 0.0634 (8) | 0.0604 (10) | 0.0672 (9) | 0.0032 (7) | 0.0177 (7) | −0.0063 (7) |
| C12 | 0.0505 (6) | 0.0480 (8) | 0.0521 (6) | 0.0086 (6) | 0.0176 (5) | 0.0059 (6) |
| C13 | 0.0471 (6) | 0.0451 (8) | 0.0719 (8) | 0.0040 (6) | 0.0252 (6) | 0.0047 (7) |
Geometric parameters (Å, °)
| N1—C1 | 1.3510 (15) | C6—H6C | 0.94 (3) |
| N1—C5 | 1.3638 (17) | O1—C7 | 1.3567 (19) |
| N1—H1N1 | 1.030 (18) | O1—H1O1 | 1.03 (2) |
| N2—C1 | 1.3279 (18) | O2—C13 | 1.2467 (15) |
| N2—H1N2 | 0.884 (18) | O3—C13 | 1.2710 (16) |
| N2—H2N2 | 0.97 (2) | C7—C8 | 1.393 (2) |
| C1—C2 | 1.405 (2) | C7—C12 | 1.4006 (18) |
| C2—C3 | 1.359 (2) | C8—C9 | 1.365 (3) |
| C2—H2A | 0.950 (15) | C8—H8A | 0.938 (19) |
| C3—C4 | 1.413 (2) | C9—C10 | 1.377 (3) |
| C3—H3A | 1.011 (18) | C9—H9A | 0.99 (2) |
| C4—C5 | 1.352 (2) | C10—C11 | 1.384 (2) |
| C4—C6 | 1.500 (2) | C10—H10A | 0.94 (2) |
| C5—H5A | 0.960 (13) | C11—C12 | 1.391 (2) |
| C6—H6A | 0.99 (2) | C11—H11A | 0.962 (15) |
| C6—H6B | 1.01 (3) | C12—C13 | 1.494 (2) |
| C1—N1—C5 | 122.26 (13) | H6A—C6—H6C | 113.4 (17) |
| C1—N1—H1N1 | 118.8 (8) | H6B—C6—H6C | 108 (2) |
| C5—N1—H1N1 | 118.9 (8) | C7—O1—H1O1 | 101.7 (11) |
| C1—N2—H1N2 | 117.6 (11) | O1—C7—C8 | 118.34 (13) |
| C1—N2—H2N2 | 118.2 (10) | O1—C7—C12 | 122.04 (13) |
| H1N2—N2—H2N2 | 124.1 (15) | C8—C7—C12 | 119.62 (15) |
| N2—C1—N1 | 118.50 (13) | C9—C8—C7 | 120.52 (16) |
| N2—C1—C2 | 123.91 (12) | C9—C8—H8A | 124.5 (13) |
| N1—C1—C2 | 117.58 (11) | C7—C8—H8A | 114.8 (13) |
| C3—C2—C1 | 119.91 (12) | C8—C9—C10 | 121.02 (18) |
| C3—C2—H2A | 122.5 (9) | C8—C9—H9A | 119.2 (11) |
| C1—C2—H2A | 117.6 (9) | C10—C9—H9A | 119.8 (11) |
| C2—C3—C4 | 121.70 (14) | C9—C10—C11 | 118.87 (18) |
| C2—C3—H3A | 121.2 (9) | C9—C10—H10A | 122.2 (13) |
| C4—C3—H3A | 117.1 (9) | C11—C10—H10A | 118.9 (13) |
| C5—C4—C3 | 116.52 (12) | C10—C11—C12 | 121.65 (15) |
| C5—C4—C6 | 121.61 (14) | C10—C11—H11A | 120.0 (10) |
| C3—C4—C6 | 121.86 (16) | C12—C11—H11A | 118.3 (9) |
| C4—C5—N1 | 122.02 (12) | C11—C12—C7 | 118.32 (13) |
| C4—C5—H5A | 126.5 (8) | C11—C12—C13 | 120.92 (12) |
| N1—C5—H5A | 111.4 (8) | C7—C12—C13 | 120.75 (12) |
| C4—C6—H6A | 111.8 (13) | O2—C13—O3 | 123.17 (13) |
| C4—C6—H6B | 108.9 (13) | O2—C13—C12 | 119.90 (12) |
| H6A—C6—H6B | 102.8 (18) | O3—C13—C12 | 116.93 (11) |
| C4—C6—H6C | 111.5 (14) | ||
| C5—N1—C1—N2 | 179.21 (12) | C8—C9—C10—C11 | −0.5 (3) |
| C5—N1—C1—C2 | −0.82 (18) | C9—C10—C11—C12 | −0.3 (3) |
| N2—C1—C2—C3 | −178.87 (13) | C10—C11—C12—C7 | 1.0 (2) |
| N1—C1—C2—C3 | 1.16 (19) | C10—C11—C12—C13 | −177.95 (14) |
| C1—C2—C3—C4 | −0.7 (2) | O1—C7—C12—C11 | 179.29 (13) |
| C2—C3—C4—C5 | −0.1 (2) | C8—C7—C12—C11 | −0.8 (2) |
| C2—C3—C4—C6 | 178.54 (15) | O1—C7—C12—C13 | −1.8 (2) |
| C3—C4—C5—N1 | 0.46 (19) | C8—C7—C12—C13 | 178.09 (13) |
| C6—C4—C5—N1 | −178.17 (14) | C11—C12—C13—O2 | −1.0 (2) |
| C1—N1—C5—C4 | 0.00 (19) | C7—C12—C13—O2 | −179.86 (12) |
| O1—C7—C8—C9 | 179.92 (15) | C11—C12—C13—O3 | 178.39 (13) |
| C12—C7—C8—C9 | 0.0 (2) | C7—C12—C13—O3 | −0.49 (19) |
| C7—C8—C9—C10 | 0.7 (3) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1—H1N1···O3 | 1.03 (2) | 1.65 (2) | 2.678 (2) | 174.6 (13) |
| N2—H1N2···O2i | 0.884 (18) | 1.987 (17) | 2.852 (2) | 165.4 (14) |
| N2—H2N2···O2 | 0.97 (2) | 1.90 (2) | 2.872 (2) | 179 (2) |
| O1—H1O1···O3 | 1.03 (2) | 1.55 (2) | 2.515 (2) | 155 (2) |
| C5—H5A···O1ii | 0.961 (14) | 2.598 (14) | 3.518 (3) | 160.2 (10) |
Symmetry codes: (i) −x, y+1/2, −z+1/2; (ii) −x+1, y+1/2, −z+1/2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: BT5314).
References
- Allen, F. H., Kennard, O., Watson, D. G., Brammer, L., Orpen, A. G. & Taylor, R. (1987). J. Chem. Soc. Perkin Trans. 2, pp. S1–19.
- Bernstein, J., Davis, R. E., Shimoni, L. & Chang, N.-L. (1995). Angew. Chem. Int. Ed. Engl.34, 1555–1573.
- Bruker (2009). APEX2, SAINT and SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
- Desiraju, G. R. (1989). Crystal Engineering: The Design of Organic Solids Amsterdam: Elsevier.
- Kvenvolden, K. A., Lawless, J. G. & Ponnamperuma, C. (1971). Proc. Natl Acad. Sci. USA, 68, 486–490. [DOI] [PMC free article] [PubMed]
- MacDonald, J. C. & Whitesides, G. M. (1994). Chem. Rev.94, 2383–2420.
- Miller, S. L. & Orgel, L. E. (1974). The Origins of Life on Earth New Jersey: Prentice Hall.
- Patel, U., Haridas, M. & Singh, T. P. (1988). Acta Cryst. C44, 1264–1267.
- Quah, C. K., Hemamalini, M. & Fun, H.-K. (2010a). Acta Cryst. E66, o1932. [DOI] [PMC free article] [PubMed]
- Quah, C. K., Hemamalini, M. & Fun, H.-K. (2010b). Acta Cryst. E66, o2164–o2165. [DOI] [PMC free article] [PubMed]
- Quah, C. K., Jebas, S. R. & Fun, H.-K. (2008). Acta Cryst. E64, o1878–o1879. [DOI] [PMC free article] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Singh, T. P. & Vijayan, M. (1974). Acta Cryst. B30, 557–562.
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536810030928/bt5314sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810030928/bt5314Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report




