Abstract
We investigated the influence of the composition of the fibrolytic microbial community on the development and activities of hydrogen-utilizing microorganisms in the rumens of gnotobiotically reared lambs. Two groups of lambs were reared. The first group was inoculated with Fibrobacter succinogenes, a non-H2-producing species, as the main cellulolytic organism, and the second group was inoculated with Ruminococcus albus, Ruminococcus flavefaciens, and anaerobic fungi that produce hydrogen. The development of hydrogenotrophic bacterial communities, i.e., acetogens, fumarate and sulfate reducers, was monitored in the absence of methanogens and after inoculation of methanogens. Hydrogen production and utilization and methane production were measured in rumen content samples incubated in vitro in the presence of exogenous hydrogen (supplemented with fumarate or not supplemented with fumarate) or in the presence of ground alfalfa hay as a degradable substrate. Our results show that methane production was clearly reduced when the dominant fibrolytic species was a non-H2-producing species, such as Fibrobacter succinogenes, without significantly impairing fiber degradation and fermentations in the rumen. The addition of fumarate to the rumen contents stimulated H2 utilization only by the ruminal microbiota inoculated with F. succinogenes, suggesting that these communities could play an important role in fumarate reduction in vivo.
Hydrogen is a major intermediary metabolite in anaerobic degradation of organic matter. In ruminants, hydrogen is produced by hydrolytic and fermentative microorganisms and is mainly used by methanogenic Archaea to reduce carbon dioxide into methane. These microorganisms represent the main ruminal microbial community implicated in this pathway (33). Hydrogen transfer through methanogenesis is beneficial to the degradation of plant cell wall carbohydrates in the rumen (19, 43, 46). However, as a result of this process, methane is eructated by ruminants (400 to 500 liters per day per adult animal) and represents a loss of carbon and energy, accounting for 8 to 12% of the gross energy content of the diet (27, 37, 44). The amount of methane produced varies according to the diet (forage or concentrate) and the production system (intensive or extensive) (27, 40). The contribution of livestock agriculture to greenhouse gas (GHG) emissions has been estimated to range between 9% and 18% of anthropogenic emissions, with methane representing between 30% and 50% of the total GHG emitted from the livestock sector (34). In that context, various strategies have been suggested in order to mitigate ruminant methane production (27, 28, 34). Reducing hydrogen production should be achieved without impairing efficacy of feed digestion and fermentation. Inhibition of activity and/or number of methanogens should be done in association with a stimulation of hydrogen-consuming pathways in order to avoid the negative effect of an increased partial pressure of this gas (28).
The aim of the present work was to investigate the effect of cellulolytic microbial populations on the development and activity of hydrogen-consuming microorganisms and methane production in the rumen. We used gnotobiotically reared lambs harboring cellulolytic species that differed in their capacity to produce hydrogen. Among the major cellulolytic communities, anaerobic fungi and ruminococci are major contributers to H2 production, whereas Fibrobacter succinogenes does not produce any hydrogen (17). F. succinogenes has been found to be the predominant cellulolytic species in the rumen of bison, and the presence of methanogens has been reported to be less important in this animal than in other ruminants, where the abundance of ruminococci and methanogens was strongly correlated (35).
MATERIALS AND METHODS
Animals and diet.
Four naturally born lambs (INRA Romane breed) from the INRA sheep flock were utilized in this study. They had been left with their dams for the first 15 to 20 h after birth before they were transferred to sterile incubators (La Calhène, Vendôme, France) where they were reared gnotobiotically (14) until they were 5 months old (end of the experiment). Two pairs of animals were studied. (i) Group FS contained two lambs inoculated with Fibrobacter succinogenes (strains S85, U, and HM2) as the sole cellulolytic organism. (ii) Group RAF contained two lambs inoculated with cellulolytic microflora composed of Ruminococcus albus 7, Ruminococcus flavefaciens FD1 and two strains of anaerobic fungi (one monocentric and one polycentric).
Until the lambs were 40 days old, they were exclusively fed cow's milk sterilized by ultrahigh temperature (UHT) processing. Once they were 40 days old, they received at libitum a diet of dehydrated alfalfa hay in 7-mm pellets (SAFE, Augy, France) that had been sterilized by gamma irradiation (4 megarads; Ionizos, Dagneux, France). Lambs were weaned after 50 days. When the animals were between 2.5 and 3 months of age, they were fitted with a permanent ruminal cannula (external diameter, 27 mm; internal diameter, 20 mm).
The experimental protocol had been reviewed and validated by the local Ethics Committee before the beginning of the experiment.
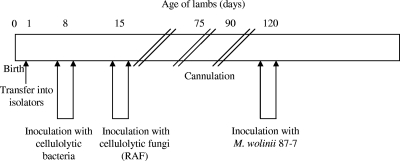
Inoculation of the different microbial communities (Fig. 1). (i) Origin of cellulolytic strains.
FIG. 1.
Timeline showing interventions according to the age of the lambs.
Fibrobacter succinogenes S85 and HM2 were obtained from the American Type Culture Collection (ATCC) (ATCC 19169 and ATCC 43856, respectively), and F. succinogenes U was provided by the Agriculture and Agri-Food Canada Lethbridge Research Centre, Lethbridge, Alberta, Canada. Ruminococcus albus 7 and Ruminococcus flavefaciens FD1 were obtained from the culture collection of the University of Illinois at Urbana-Champaign.
Ruminal fungal strains were isolated in our laboratory from a hay-fed sheep with a cannula in its rumen. On the basis of microscopic observation, a monocentric strain (presumptively close to Neocallimastix frontalis) and a polycentric strain (morphologically close to Orpinomyces joyonii) were selected from bacterium-free culture enrichments in the medium of Orpin (38) supplemented with a mixture of antibiotics (8) and a strip of filter paper (Whatman no. 1).
All microbes were cultured under strictly anaerobic conditions (23).
(ii) Inoculation with cellulolytic strains.
The absence of cellulolytic bacteria and fungi in each lamb rumen before inoculation with pure cultures was carefully checked by inoculating triplicate Hungate tubes containing culture medium and filter paper cellulose strips with 10−1, 10−2, and 10−3 dilutions of fresh rumen contents, obtained by stomach tubing, from the 2-day-old and 5-day-old lambs. The tubes were incubated at 39°C for at least 2 weeks, and we checked that the filter paper strips remained totally undegraded.
The cellulolytic bacterial inocula were prepared in a ruminal-fluid-based anaerobic medium (20). After 24 h of incubation at 39°C, one 10-ml tube containing a mixture of F. succinogenes strains or one 10-ml tube containing a mixture (50:50) of both R. albus and R. flavefaciens were inoculated into the 8-day-old lambs for three consecutive days. The RAF lambs were inoculated with fungal cultures over a period of three consecutive days 1 week later. One 10-ml culture tube containing a mixture (50:50) of each fungal isolate grown for 24 to 48 h in a ruminal-fluid- and cellobiose-based medium was given to each lamb per day using a stomach polyvinyl chloride (PVC) tube (external diameter, 6 mm; internal diameter, 3 mm).
(iii) Inoculation of the methanogenic strain.
When the animals were 120 days old, they all received an inoculum of strain 87-7, a methanogenic strain isolated from lamb rumen in our laboratory (F. Chaucheyras-Durand, unpublished data). On the basis of morphological and metabolic criteria, this strain was related to a Methanobrevibacter species. Sequencing of the 16S rRNA gene (EMBL accession number AM269413) indicated that this strain was phylogenetically close to Methanobrevibacter wolinii (98% identity). The contents of one culture tube containing the 87-7 strain, grown on a complex medium (3) under a pressure of 202 kPa in a mixture of H2 and CO2 (80:20), was administered to the animals during three consecutive days through the cannula in the rumen.
Microbiological analyses.
Samples from the rumen were collected before the morning feeding by aspiration with a stomach tube in lambs less than 3 months old and through the cannula afterwards. Serial dilutions of each sample were prepared in the anaerobic mineral solution (6). These dilutions were then utilized for enumeration of different microbial functional groups, i.e., total viable anaerobic bacteria, cellulolytic bacteria, anaerobic fungi, and methanogenic Archaea, according to previously described methods (15).
Acetogenic bacteria, methanogenic Archaea, and sulfate-reducing bacteria (SRB) were enumerated in specific liquid media by the methods of Doré et al. (11) and Morvan et al. (35, 36) after incubation at 39°C in a H2-CO2 (80:20) atmosphere (202 kPa) for 2 to 3 weeks. Fumarate-reducing bacteria (FRB) were enumerated in the Wolinella succinogenes medium of Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ) (DSMZ medium number 157) modified by the method of Fonty et al. (16). Cultures were incubated for 3 weeks at 39°C in a H2-N2 (50:50) atmosphere (202 kPa). Growth of FRB was considered to occur when a decrease in gas pressure (compared to uninoculated pressurized tubes) together with an increase in succinate and/or propionate were observed. Bacterial numbers were estimated according to the most-probable-number method (9).
Degradation of alfalfa hay in bags in the rumen.
Fiber degradation in the rumens of all lambs was determined before and after inoculation of methanogens (at 100 and 140 days of age, respectively) by the nylon bag method (31). Each bag (pore size, 50 μm) contained 0.8 g of ground alfalfa pellets. The bags were incubated for periods of 24 and 48 h in the rumen, and the incubations were repeated four times for each lamb. After incubation, the bags were removed from the rumen, washed with tap water, dried at 70°C, and weighed. The amount of alfalfa hay digested was then estimated by the percentage loss of dry matter (DM).
Hydrogen utilization by hydrogenotrophic communities.
We estimated the hydrogen-utilizing capacities of the ruminal hydrogenotrophic populations harbored by the different animal models. To achieve this goal, we performed in vitro incubations of the contents of the rumen in the presence of hydrogen either produced from alfalfa hay degradation and fermentation or added as an exogenous substrate as described previously (13, 16). Rumen content samples (approximately 120 g) were obtained at the following times: 2 h after the morning feeding, from each lamb when they were 98 to 100 days old, i.e., before inoculation of methanogens, and when they were 138 to 140 days old, i.e., after inoculation of methanogens. The samples were filtered through two layers of cheesecloth, and 25-ml portions of filtered rumen samples were diluted in 15 ml of anaerobic mineral solution (6) and incubated in vitro for 0, 24, and 48 h in 125-ml flasks under the following gaseous atmospheres and conditions: 100% CO2 plus 200 mg ground alfalfa pellets, 202 kPa of H2-CO2 (80:20), 202 kPa of H2-N2 (50:50), or 202 kPa of H2-N2 (50:50) plus 30 mM sodium fumarate.
At the beginning and end of incubation, total gas volume in each flask was measured by displacement using a glass syringe, and gas composition was determined by gas chromatography. Five milliliters of each sample was also centrifuged (1,000 × g, 15 min, 4°C), and supernatants were used for determination of the short-chain fatty acid (SCFA) concentration by one-dimensional (1D) 1H nuclear magnetic resonance (NMR) analysis (29).
Statistical analyses.
In the figures and tables, results are given as means with their standard deviations (SDs). Data were analyzed with one-way analysis of variance, and differences between groups were considered to be significant at P < 0.10 using Student's t test.
RESULTS
Establishment of microbial communities in the rumens of gnotobiotically reared lambs.
Total anaerobic bacteria rapidly established at high levels in all lambs; 5 days after birth, this community already ranged between 1.6 × 109 and 7.4 × 109 CFU·ml−1 and slightly increased during the first weeks. No significant differences were observed for total bacterial counts within the two groups of animals.
Cellulolytic bacteria were not detected in the rumen of any lamb before inoculation of the different species. In both groups, they began to be detected a few days after their inoculation with pure cultures. The respective population mean sizes were 2.5 × 107 and 5.0 × 106 cells·ml−1 for FS and RAF lambs before inoculation of methanogens and 5.0 × 107 and 1.6 × 106 cells·ml−1 after inoculation of methanogens.
Cellulolytic fungi were never detected in the rumens of FS lambs and not detected in the rumens of RAF lambs before their inoculation with pure cultures. After their inoculation, they were detected consistently in the rumen of only one lamb from the RAF group, whereas they were found only sporadically in the rumen of the other RAF lamb; zoospore concentrations ranged between 102 and 103·ml−1 of rumen contents.
Methanobrevibacter wolinii-related strain 87-7 established rapidly in the rumens of all lambs. The mean number of strain 87-7 bacteria was close to 106 cells·ml−1 1 week after inoculation and increased thereafter to reach mean values above 5 × 107 cells·ml−1.
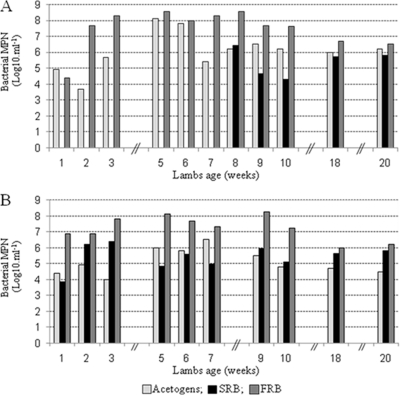
In FS lambs, in the absence of methanogens, acetogenic bacteria were detected in the rumen at low levels just after Fibrobacter succinogenes inoculation, then the size of the population increased to ∼108 bacteria·ml−1 (Fig. 2A). After 6 weeks, it fell to lower levels. In the presence of methanogens, only one sample per lamb was analyzed, and the population was close to 106 cells·ml−1. Sulfate-reducing bacteria (SRB) were detected only after 50 days in the rumens of FS lambs; the numbers of bacteria were rarely above 106 cells·ml−1.
FIG. 2.
Ruminal concentrations of acetogens, sulfate-reducing bacteria (SRB), and fumarate-reducing bacteria (FRB) according to the age of the lambs. (A) FS lambs; (B) RAF lambs. For each time point, the results are the means of two most-probable-number (MPN) determinations in each group.
In RAF lambs, acetogenic bacteria were detected soon after birth, but their population size rarely reached 106 bacteria·ml−1 (Fig. 2B). After inoculation of methanogens, acetogen concentrations decreased in the rumens of RAF lambs and were detected only at low levels (3.0 × 104 to 4.9 × 104·ml−1). SRB were present very soon after birth. Their population size stabilized between 105 and 106 bacteria·ml−1 even after establishment of methanogens.
In both FS and RAF lambs, fumarate-reducing bacteria (FRB) were detected soon after birth at a concentration close to 107 cells·ml−1. This concentration remained stable, between 107 and 108 bacteria·ml−1 in the absence of methanogens. Although only one ruminal fluid sample from each animal could be analyzed after inoculation of M. wolinii, we noticed that the FRB population tended to decrease (1-log-unit decrease) when methanogens were present in the eco- system.
Fiber degradation in bags in the rumen.
The percentages of alfalfa hay dry matter (DM) degradation were close to 50% after 24 h of incubation (Table 1). DM degradation was not statistically significantly different between the two groups.
TABLE 1.
Disappearance of alfalfa hay dry matter in bags in the rumens of gnotobiotically reared lambs before and after establishment of methanogens
| Animal model | Methanogenic statusa | DM disappearanceb (%) in samples incubated for the following time: |
|
|---|---|---|---|
| 24 h | 48 h | ||
| FS lambs | Absent | 49.9 ± 2.1 | 50.3 ± 3.6 |
| Present | 51.9 ± 3.6 | 53.6 ± 3.3 | |
| RAF lambs | Absent | 53.5 ± 4.4 | 53.9 ± 2.5 |
| Present | 57.4 ± 5.5 | 57.8 ± 6.4 | |
Absent, methanogens absent; Present, methanogens present.
The disappearance of dry matter (DM) is shown. The values are means ± standard deviations (SDs) for at least 4 values.
In vitro hydrogen production and consumption and methane production. (i) Short-chain fatty acid (SCFA) concentrations in lamb rumen contents.
Before incubation of the flasks, SCFA concentrations were determined in the ruminal fluid samples obtained 2 h after feeding. The concentrations of the main SCFAs (acetate, propionate, and butyrate) were not significantly different in the two groups of animals (Table 2). However, the composition of the SCFA mixture varied. Before inoculation of strain 87-7, we observed a high percentage of butyrate in the samples from FS lambs and a high percentage of acetate and propionate in the samples from RAF lambs. After inoculation with methanogens, acetate proportion increased in FS lambs and propionate and butyrate proportions sharply decreased, whereas no great changes in the SCFA proportions were observed in RAF lambs.
TABLE 2.
Main short-chain fatty acid concentrations and proportions in ruminal fluid samples of gnotobiotically reared lambsa
| Animal model | Methanogenic statusb | Concn of the main SCFAsc (mmol·liter−1) | Proportion (%) of the following SCFA in ruminal fluid samples |
||
|---|---|---|---|---|---|
| Acetate | Propionate | Butyrate | |||
| FS lambs | Absent | 95.5 ± 18.3 | 71.2 | 17.9 | 10.9 |
| Present | 102.1 ± 14.2 | 84.0 | 9.2 | 6.5 | |
| RAF lambs | Absent | 87.6 ± 11.4 | 77.7 | 19.8 | 2.6 |
| Present | 95.0 ± 16.4 | 75.6 | 21.3 | 3.1 | |
The samples were obtained 2 h after the morning feeding in the absence or presence of methanogens.
Absent, methanogens absent; Present, methanogens present.
The main short-chain fatty acids (SCFAs) were acetate, propionate, and butyrate. The values are means ± standard deviations (SDs) for at least 4 values.
(ii) Incubations in the presence of ground alfalfa hay and 100% CO2 in the gas phase.
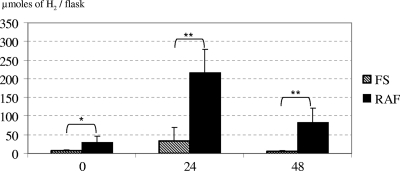
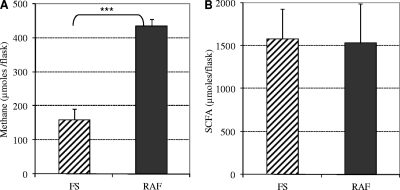
When alfalfa was added to the flasks containing rumen contents taken before establishment of methanogens, H2 production peaked after 24 h of incubation and decreased thereafter (Fig. 3). Methane was never detected in the gas phase. Significantly higher H2 quantities were produced from alfalfa fermentation by ruminal fluid samples from RAF lambs than by samples from FS lambs. After inoculation of methanogens, almost no hydrogen was recovered, and methane was detected in the flasks (Fig. 4A). The amount of methane produced from RAF lamb rumen contents was significantly greater than that produced from FS lamb rumen contents after 48 h of incubation, whereas no significant difference in major SCFA production was measured between the two lamb groups (Fig. 4B). Moreover, the amount of methane produced during incubation of samples from RAF lambs exceeded the amount expected to be generated from H2 produced from alfalfa, based on H2 quantities measured before inoculation of strain 87-7 and on stoichiometry of methanogenesis pathway (4 mol H2 necessary to synthesize 1 mol CH4).
FIG. 3.
Hydrogen production from in vitro incubations of ruminal fluid samples obtained from the two groups of lambs (FS and RAF lambs). The samples were incubated in the presence of ground alfalfa. Values represent means plus standard deviations (SDs) (error bars) from two values for each lamb within each group. Within the same incubation time, values for samples from the two groups were significantly different as follows: *, P < 0.05; **, P < 0.01.
FIG. 4.
In vitro methane (A) and major short-chain fatty acid (SCFA) (B) production after 48 h of incubation of rumen contents from both groups of lambs in the presence of ground alfalfa after establishment of M. wolinii 87-7. The values are the means of two values per lamb with their SDs. The values for the two groups were significantly different (P < 0.001) as indicated by the three asterisks.
(iii) Incubations with H2-CO2.
In FS lambs, in the absence of methanogens, and when exogenous hydrogen was introduced in the headspace using a H2-CO2 mixture, approximately 78% of the initial H2 was consumed within 48 h (Table 3). Methane was never detected. Ninety-eight percent of H2 was consumed after 48 h of incubation of ruminal fluid obtained from the FS lambs 12 days after inoculation with the methanogenic strain, but based on the stoichiometry of methanogenesis, the calculated percentage of H2 actually required for methane formation was only 47% (data not shown). However, when the ruminal fluid samples of FS lambs were obtained 27 days after inoculation of methanogens, all H2 was accounted for by methane formation (Table 4).
TABLE 3.
Utilization of H2 after 48 h of incubation of mixed ruminal bacteria of the two groups of lambs with different gas mixtures before and after inoculation of methanogens
| Animal model | Methanogenic statusa | H2 utilization (%)b by mixed ruminal bacteria under the following conditions: |
||
|---|---|---|---|---|
| H2-CO2 | H2-N2 | H2-N2 + fumarate | ||
| FS lambs | Absent | 77.9 | 37.0 | 60.6 |
| Present | 98.0 | 87.9 | 94.1 | |
| RAF lambs | Absent | 48.7 | 41.9 | 42.6 |
| Present | 96.8 | 86.5 | 99.6 | |
Absent, methanogens absent; Present, methanogens present.
Utilization of H2 is shown as a percentage of the initial concentration at the beginning of incubation of two flasks per animal.
TABLE 4.
Hydrogen consumed and methane produced after incubation of the contents of rumens from the two groups of lambsa
| Incubation condition(s) | Animal model | Amt of H2 consumed (μmol/flask) | Amt of CH4 produced (μmol/flask) |
||
|---|---|---|---|---|---|
| Expectedb | Measured | % Recovery | |||
| H2-CO2 | FS lambs | 4,960 | 1,240 | 1,234 | 99.5 |
| RAF lambs | 5,290 | 1,323 | 1,459 | 110.3 | |
| H2-N2, without fumarate | FS lambs | 6,561 | 1,640 | 1,686 | 103.0 |
| RAF lambs | 5,860 | 1,465 | 1,455 | 99.3 | |
| H2-N2, with fumarate | FS lambs | 6,030 | 1,508 | 999 | 66.2 |
| RAF lambs | 6,148 | 1,537 | 1,881 | 122.4 | |
Ruminal fluid samples from the lambs were incubated for 48 h in H2-CO2 or H2-N2 atmosphere in the presence or absence of fumarate after establishment of methanogens.
The amount of methane expected has been calculated from stoechiometry of the reaction 4H2 + CO2 → CH4 + 2H2O.
In the absence of methanogens, hydrogen utilization by the ruminal microflora of RAF lambs represented 48.7% of the initial amount within 48 h (Table 3). Methane was not recovered in the gas phase. In the presence of a stable population of methanogens, 96.8% of H2 was removed from the flasks within 48 h of incubation, and all of it was involved in the methanogenesis pathway (Table 4).
(iv) Incubations in an atmosphere of H2-N2 with or without fumarate.
When the contents of the rumens of FS lambs were incubated under a H2-N2 atmosphere, in the absence of M. wolinii 87-7, hydrogen consumption was lower than when H2-CO2 was used as the gas mixture (Table 3). Indeed, only 37% of the H2 was used within 48 h. When fumarate was added to the incubation medium, the percentage of H2 utilization by the flora increased to 60.6%. Methane was never detected in the gas phase.
Almost 42% of the H2 was consumed within 48 h by the microflora of RAF lambs in the absence of methanogens; however, this percentage was not increased by the addition of fumarate, in contrast to what was observed with FS rumen samples (Table 3).
After establishment of methanogens, in the absence of fumarate, a sharp increase in H2 utilization was observed in samples from FS and RAF lambs; in the presence of fumarate, almost 100% H2 was consumed after 48 h of incubation of samples from both groups of lambs, but the recovery of methane produced related to the amount of hydrogen consumed was only 66% in FS samples, whereas it was a little higher than 100% in RAF samples (Table 4).
DISCUSSION
Establishment of microbial populations in the rumens of gnotobiotically reared lambs.
We used animal models with different fibrolytic species to better understand the relationships between hydrogen-producing and -consuming communities in the absence of methanogens and after inoculation of methanogens. Considering the poor knowledge of the physiology and metabolism of these communities, these animal models appeared particularly relevant to study these kinds of interactions. In these lambs, rumen bacterial and fungal population densities were found to be close to those reported for conventionally reared animals (15, 18).
The detection of acetogens very soon after birth was in accordance with previous findings from conventionally reared animals (16, 36). Indeed, it is very likely that colonization of the rumen by these communities occurs thanks to maternal transmission. Because of their metabolic versatility, acetogenic bacteria can use a wide range of substrates for growth in addition to H2-CO2 (12). Our enumeration method being based only on the capacity of H2-CO2 uptake, we could have underestimated the population density of acetogens as has been discussed elsewhere (16). A quantitative real-time PCR approach targeting a functional fhs gene encoding the formyltetrahydrofolate synthetase (FTHFS), a key enzyme implicated in reductive acetogenesis pathway, has been proposed (47) but has not been yet applied and validated for digestive ecosystems.
SRB were not detected in the rumens of FS lambs before 50 days (weaning period). This may be due to a low availability of hydrogen in the rumens inoculated with F. succinogenes. Although FRB population densities were found in accordance with other studies (16), they were quite variable, and consequently, it is not possible to know whether the nature of fibrolytic communities influences its development.
After establishment of M. wolinii 87-7, only a few samples were analyzed for bacterial enumeration, which made it difficult to reach a conclusion regarding its impact on other bacterial communities. We did not observe a significant modification of the cellulolytic bacterial community size, but we noticed fluctuations of acetogenic bacterial numbers, which declined in the rumens of RAF lambs. FRB also tended to decrease slightly in all groups after methanogen inoculation. These data confirm that methanogenic Archaea impact negatively on other hydrogen-consuming populations, even those established for a long time, particularly acetogenic bacterial communities which are known to have a higher H2 utilization threshold than methanogens do (10).
Fiber degradation.
In our animal models, the relatively high percentages of DM degradation illustrate an efficacious fibrolytic function in the rumens of both groups of lambs. The slight improvement of fiber degradation observed after establishment of methanogens is in accordance with previous findings in a similar animal model (19). Indeed, through interspecies hydrogen transfer, methanogens contribute to a low H2 partial pressure, which is essential for optimal functioning of hydrolytic microorganisms. Considering the same methanogenic status, no significant differences were obtained between the two groups of lambs. However, the slightly better degradation noticed in the rumens of RAF lambs may be explained by the effect of efficient enzymatic equipment of both ruminococci and fungi and the mechanical activity of fungal rhizoids (45), although competitive interactions between ruminococci and fungi have been described in vitro (5).
Hydrogen production and utilization in the absence of methanogens.
When ruminal fluid samples were incubated with alfalfa hay as the substrate, no exogenous gas was added to the flasks, and we measured only small amounts of hydrogen as a result of simultaneous production-uptake processes. However, accumulation of H2 was observed only in the samples from RAF lambs after 24 h of incubation, suggesting that promoting non-H2-producing fibrolytic bacteria such as F. succinogenes limits hydrogen disposal for hydrogenotrophic populations. The fact that some H2 remained in flasks containing ruminal fluid samples from RAF lambs suggested that establishing H2-producing microorganisms as the dominant fibrolytic microflora does not induce a greater capacity of H2 utilization by hydrogenotrophic communities.
Addition of different gaseous mixtures to incubated flasks allowed us to focus on hydrogen-consuming activities of the different hydrogenotrophic communities present in the rumens of the two animal models. In the presence of H2-CO2, an efficient hydrogen-consuming bacterial population in the rumens of FS lambs used H2, possibly through the reductive acetogenesis pathway; however, acetate constituted only 60% of the major SCFA mixture (data not shown), which suggests that this activity might be limited; indeed, the presence of F. succinogenes as the sole fibrolytic bacterial species could limit the activity of these hydrogenotrophic populations. Hydrogen could also be utilized through other routes. Among the bacterial consortia preferentially associated with F. succinogenes, Butyrivibrio fibrisolvens representatives have been identified (41). In ruminal fluid samples from FS lambs obtained 2 h after feeding and in 48-h incubations in a H2-CO2 atmosphere, butyrate represented a large proportion of the SCFA mixture (10.9 and 20%, respectively), which would support the hypothesis of a high prevalence of B. fibrisolvens. This species is involved in the fatty acid biohydrogenation pathway, and it may contribute to hydrogen uptake. However, this reaction is likely to play a rather minor role in hydrogen consumption in vivo (25). Acetate might also be reutilized for butyrogenesis by B. fibrisolvens or other species, which could explain the low levels of acetate.
In RAF lambs, the presence of hydrogen producers such as ruminococci and fungi as cellulose-degrading organisms was likely to favor the H2-consuming activity, but it is noteworthy that the rate of H2 consumed was lower than that measured in incubated flasks with ruminal fluid samples from FS lambs, which was surprising. One possible explanation might be the production of reduced components by the ruminococci and fungi, such as formate, which was detected at the beginning of incubation (5.3 ± 1.4 mM) but which was not found after 48 h of incubation. Formate might have been used, instead of H2, as an electron donor for hydrogen-consuming bacteria. However, the capacity of hydrogen utilization by acetogens appeared to be higher than that of fumarate-reducing bacteria, because the percentage of H2 utilization in a H2-N2 atmosphere in both animal models was only around 40%. This observation suggests that reductive acetogenesis would represent an important hydrogen sink in the rumen and that it would be interesting to take strategies aiming at favoring this pathway into consideration (16, 24).
The fact that adding fumarate to incubated flasks containing ruminal fluid samples from lambs inoculated with F. succinogenes induced a significant increase in hydrogen utilization indicates that the microbiota containing F. succinogenes can use fumarate efficiently and increase the removal of H2. This was supported by NMR data showing a 48% increase in propionate proportion in flasks supplemented with fumarate, which suggested that the fumarate reduction pathway was activated, generating succinate being decarboxylated into propionate by several bacterial species (17). The results of in vitro studies have suggested that F. succinogenes is able to reduce fumarate to succinate by using H2 as an electron donor (1, 2), but earlier papers reported that fumarate reduction by F. succinogenes was achieved only in the presence of NADH or the reduced form of flavin mononucleotide (FMNH2) (30, 32). Therefore, it cannot be concluded that the increase of H2 utilization was due to the activity of F. succinogenes alone. However, other bacterial species such as Wolinella succinogenes, Anaerovibrio lipolytica, and Selenomonas ruminantium have been reported to use hydrogen as an electron donor to reduce fumarate (22, 26, 42). Clones related to several clusters within the Proteobacteria phylum, possessing the frdA gene encoding the α-subunit of fumarate reductase, have also been characterized (21). These species, potentially present in the rumen contents of our lambs, could also be implicated in hydrogen removal from the incubated flasks. Recent findings (41) indicate that different bacterial consortia may be associated with different subgroups of F. succinogenes strains. In this context, further studies are needed to better characterize the composition of the microbial communities harbored by the FS lambs.
Hydrogen utilization in the presence of methanogens.
In most of our in vitro experiments and regardless of the composition of fibrolytic communities, the proportion of hydrogen required for methanogenesis was not very important just after inoculation with M. wolinii 87-7, indicating that non-CH4-producing hydrogenotrophs were able to compete with methanogens for H2 utilization at least during the period of establishment of M. wolinii in the rumen. When ruminal fluid samples obtained once the methanogens had stabilized (1 week to 10 days after inoculation) were incubated, almost all the hydrogen was involved in methane production, which confirms the dominance of methanogens in the ecological niche for H2 utilization (16), because of higher substrate affinity and lower thresholds for H2 uptake (12). These results also suggest that to express their complete potential of H2 utilization, methanogens need some time to adapt to the ecosystem.
When ruminal fluid samples were incubated with alfalfa hay as the substrate, methane production was significantly higher when the microflora contained ruminococci and fungi instead of F. succinogenes, but at the same time, there was no significant difference in SCFA content in the rumens of the two groups. The hydrogen production status of the fibrolytic community could influence methane synthesis in the rumen. These data corroborate with earlier results from Morvan et al. (35), who observed that in the rumens of bison, F. succinogenes was largely dominant among the cellulolytic bacterial populations and that methanogenic Archaea densities were lower than in the rumens of other domestic ruminants. Similarly, in the feces of methane-excreting human subjects (carrying methanogens in their colon), the cellulolytic bacterial isolates were hydrogen-producing Ruminococcus and Enterococcus species (39), whereas in the feces of non-methane excretors, the cellulolytic isolates were related to non-H2-producing Bacteroides species (7).
After inoculation of methanogens and in the presence of fumarate in the flasks containing ruminal fluid samples from FS lambs, the recovery of methane produced/allowed by hydrogen disposal was only 66%, suggesting an active contribution of the microbiota containing F. succinogenes in the fumarate reduction process.
Conclusion.
Using an original animal model requiring gnotobiology techniques, we show here that the composition of the cellulolytic community (hydrogen producers versus nonhydrogen producers) may have an impact on hydrogen accumulation and subsequent methane production in the rumen ecosystem. When the animal is young, the promotion of fibrolytic organisms which do not produce any hydrogen such as Fibrobacter succinogenes, with some control of methanogen activity, would represent an interesting ecological means to help limit methane emissions in the rumen. Feed additives such as selected direct-fed microbials able to enhance growth or/and activity of this bacterial species, together with fumarate supplementation, would be worth further study.
Acknowledgments
This work was done with financial support from Lallemand Animal Nutrition.
We thank Anne-Marie Delort for providing access to the NMR spectrometer and Régis Egrot for skilled technical assistance. We are also very grateful to Gérard Vert, Christophe de Martrin, and Michel Chavarot for rearing lambs and to Rémy Roux for help with gas chromatography (GC) analyses.
Footnotes
Published ahead of print on 22 October 2010.
REFERENCES
- 1.Asanuma, N., and T. Hino. 2000. Activity and properties of fumarate reductase in ruminal bacteria. J. Gen. Appl. Microbiol. 49:119-125. [DOI] [PubMed] [Google Scholar]
- 2.Asanuma, N., M. Iwamoto, and T. Hino. 1999. Effect of the addition of fumarate on methane production by ruminal microorganisms in vitro. J. Dairy Sci. 82:780-787. [DOI] [PubMed] [Google Scholar]
- 3.Balch, W. E., G. E. Fox, L. J. Magrum, C. R. Woese, and R. S. Wolfe. 1979. Methanogens: reevaluation of a unique biological group. Microbiol. Rev. 43:260-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reference deleted.
- 5.Bernalier, A., G. Fonty, F. Bonnemoy, and P. Gouet. 1993. Inhibition of the cellulolytic activity of Neocallimastix frontalis by Ruminococcus flavefaciens. J. Gen. Microbiol. 139:873-880. [DOI] [PubMed] [Google Scholar]
- 6.Bryant, M. P., and L. A. Burkey. 1953. Cultural methods and some characteristics of some of the more numerous groups of bacteria in the bovine rumen. J. Dairy Sci. 36:205-217. [Google Scholar]
- 7.Chassard, C., B. Gaillard-Martinie, and A. Bernalier-Donadille. 2005. Interaction between H2-producing and non-H2-producing cellulolytic bacteria from the human colon. FEMS Microbiol. Lett. 242:339-344. [DOI] [PubMed] [Google Scholar]
- 8.Chaucheyras, F., G. Fonty, and G. Bertin. 1995. Effects of live Saccharomyces cerevisiae cells on zoospore germination, growth, and cellulolytic activity of the rumen anaerobic fungus, Neocallimastix frontalis MCH3. Curr. Microbiol. 31:201-205. [DOI] [PubMed] [Google Scholar]
- 9.Clarke, K. R., and N. J. P. Owens. 1983. A simple and versatile micro-computer program for the determination of “most probable number.” J. Microbiol. Methods 1:133-137. [Google Scholar]
- 10.Cord-Ruwisch, R., H. J. Seitz, and R. Conrad. 1988. The capacity of hydrogenotrophic anaerobic bacteria to compete for traces of hydrogen depends on the redox potential of the terminal electron acceptor. Arch. Microbiol. 149:350-357. [Google Scholar]
- 11.Doré, J., B. Morvan, F. Rieu-Lesme, I. Goderel, P. Gouet, and P. Pochart. 1995. Most probable number enumeration of H2-utilizing acetogenic bacteria from the digestive tract of animals and man. FEMS Microbiol. Lett. 130:7-12. [DOI] [PubMed] [Google Scholar]
- 12.Drake, H. L., K. Küsel, and C. Matthies. 2002. Ecological consequences of the phylogenetic and physiological diversities of acetogens. Antonie van Leeuwenhoek 81:203-213. [DOI] [PubMed] [Google Scholar]
- 13.Fonty, G., M. Chavarot, Y. Mille, F. Michallon, and F. Chaucheyras-Durand. 1999. Establishment of hydrogenotrophic acetogenic bacteria in the rumen of gnotobiotically-reared lambs. Microecol. Ther. 28:105-114. [Google Scholar]
- 14.Fonty, G., P. Gouet, and J. M. Nebout. 1989. Development of the cellulolytic microflora in the rumen of lambs transferred into sterile isolators a few days after birth. Can. J. Microbiol. 35:416-422. [DOI] [PubMed] [Google Scholar]
- 15.Fonty, G., P. Gouet, J. P. Jouany, and J. Senaud. 1987. Establishment of the microflora and anaerobic fungi in the rumen of lambs. J. Gen. Microbiol. 133:1835-1843. [Google Scholar]
- 16.Fonty, G., K. N. Joblin, M. Chavarot, R. Roux, and F. Michallon. 2007. Methanogen-free lambs: establishment and development of ruminal hydrogenotrophs. Appl. Environ. Microbiol. 73:6391-6403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fonty, G., J. P. Jouany, E. Forano, and P. Gouet. 1995. L'écosystème microbien du réticulo-rumen, p. 299-347. In R. Jarrige (ed.), Nutrition des ruminants domestiques. INRA, Paris, France.
- 18.Fonty, G., J. Senaud, J. P. Jouany, and P. Gouet. 1988. Establishment of ciliate protozoa in the rumen of conventional and conventionalized lambs: influence of diet and management conditions. Can. J. Microbiol. 34:235-241. [DOI] [PubMed] [Google Scholar]
- 19.Fonty, G., A. G. Williams, F. Bonnemoy, B. Morvan, S. E. Withers, and P. Gouet. 1997. Methanobrevibacter sp. MF1 inoculation on glycoside hydrolase and polysaccharide depolymerase activities, wheat straw degradation and volatile fatty acid concentrations in the rumen of gnotobiotically-reared lambs. Anaerobe 3:383-389. [DOI] [PubMed] [Google Scholar]
- 20.Halliwell, G., and M. P. Bryant. 1963. The cellulolytic activity of pure strains of bacteria from the rumen of cattle. J. Gen. Microbiol. 32:441-448. [DOI] [PubMed] [Google Scholar]
- 21.Hattori, K., and H. Matsui. 2008. Diversity of fumarate-reducing bacteria in the bovine rumen revealed by culture dependent and independent approaches. Anaerobe 14:87-93. [DOI] [PubMed] [Google Scholar]
- 22.Henderson, C. 1980. The influence of extracellular hydrogen on the metabolism of Bacteroides ruminicola, Anaerovibrio lipolytica and Selenomonas ruminantium. J. Gen. Microbiol. 119:485-491. [DOI] [PubMed] [Google Scholar]
- 23.Hungate, R. E. 1966. The rumen and its microbes. Academic Press, New York, NY.
- 24.Joblin, K. N. 1999. Ruminal acetogens and their potential to lower ruminant methane emissions. Aust. J. Agric. Res. 50:1307-1313. [Google Scholar]
- 25.Kobayashi, T. 2010. Abatement of methane production from ruminants: trends in the manipulation of rumen fermentation. Asian-Aust. J. Anim. Sci. 23:410-416. [Google Scholar]
- 26.Kröger, A., S. Biel, J. Simon, R. Gross, G. Unden, and C. R. D. Lancaster. 2002. Fumarate respiration of Wolinella succinogenes: enzymology, energetics and coupling mechanism. Biochim. Biophys. Acta 1553:22-28. [DOI] [PubMed] [Google Scholar]
- 27.Martin, C., D. P. Morgavi, M. Doreau, and J. P. Jouany. 2006. Comment réduire la production de méthane chez les ruminants? Fourrages 187:283-300. [Google Scholar]
- 28.Martin, C., M. Doreau, and D. P. Morgavi. 2008. Methane mitigation in ruminants: from rumen microbes to the animal, p. 130-133. In P. Rowlinston, M. Steele, and A. Nefzaoui (ed.), Livestock and global climate change. Proceedings of the International Conference on Livestock and Global Climate Change 2008. British Society of Animal Science, Midlothian, United Kingdom.
- 29.Matulova, M., R. Nouaille, P. Capek, P. Péan, E. Forano, and A. M. Delort. 2005. Degradation of wheat straw by Fibrobacter succinogenes S85: a liquid and solid nuclear magnetic resonance study. Appl. Environ. Microbiol. 71:1247-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meinhardt, S. W., and T. L. Glass. 1994. NADH-linked fumarate reductase and NADH dehydrogenase activities in Fibrobacter succinogenes. Curr. Microbiol. 28:247-251. [Google Scholar]
- 31.Michalet-Doreau, B. 1990. Use of in sacco method to predict the feeding values of forages, p. 1850-1852. Proceedings of the 16th International Grass Congress, Nice, France, vol. 3. INRA, Paris, France. [Google Scholar]
- 32.Miller, T. L. 1978. The pathway of formation of acetate and succinate from pyruvate by Bacteroides succinogenes. Arch. Microbiol. 117:145-152. [DOI] [PubMed] [Google Scholar]
- 33.Miller, T. L. 1995. Ecology of methane production and hydrogen sinks in the rumen, p. 317-331. In W. V. Engelhardt, S. Leonhard-Marek, G. Breves, and D. Giesecke (ed.), Ruminant physiology: digestion, metabolism, growth and reproduction. Ferdinand Enke Verlag, Stuttgart, Germany.
- 34.Morgavi, D. P., E. Forano, C. Martin, and C. J. Newbold. 2010. Microbial ecosystem and methanogenesis in ruminants. Animal 4:1024-1036. [DOI] [PubMed] [Google Scholar]
- 35.Morvan, B., F. Bonnemoy, G. Fonty, and P. Gouet. 1996. Quantitative determination of H2-utilizing acetogenic and sulfate reducing bacteria and methanogenic archaea from digestive tract of different mammals. Curr. Microbiol. 32:129-133. [DOI] [PubMed] [Google Scholar]
- 36.Morvan, B., J. Doré, F. Rieu-Lesme, L. Foucat, G. Fonty, and P. Gouet. 1994. Establishment of hydrogen-utilizing bacteria in the rumen of the newborn lamb. FEMS Microbiol. Lett. 117:249-256. [DOI] [PubMed] [Google Scholar]
- 37.Moss, A. R., J. P. Jouany, and C. J. Newbold. 2000. Methane production by ruminants: its contribution to global warming. Ann. Zootech. 49:231-253. [Google Scholar]
- 38.Orpin, C. G. 1975. Studies on the rumen flagellate Neocallimastix frontalis. J. Gen. Microbiol. 91:249-262. [DOI] [PubMed] [Google Scholar]
- 39.Robert, C., C. Del'Homme, and A. Bernalier-Donadille. 2001. Interspecies H2 transfer in cellulose degradation between fibrolytic bacteria and H2-utilizing microorganisms from the human colon. FEMS Microbiol. Lett. 205:209-214. [DOI] [PubMed] [Google Scholar]
- 40.Sauvant, D., J. P. Jouany, S. Giger-Reverdin, M. Vermorel, and G. Fonty. 1999. Production de CH4 par les ruminants: analyse des processus, quantification et modélisation, spatialisations et bilans, possibilités de réduction des émissions. C. R. Acad. Agric. France 85:70-86. [Google Scholar]
- 41.Shinkai, T., T. Ueki, and Y. Kobayashi. 2010. Detection and identification of rumen bacteria constituting a fibrolytic consortium dominated by Fibrobacter succinogenes. Anim. Sci. J. 81:72-79. [DOI] [PubMed] [Google Scholar]
- 42.Ungerfeld, E. M., R. A. Kohn, R. J. Wallace, and C. J. Newbold. 2007. A meta-analysis of fumarate effects on methane production in ruminal batch cultures. J. Anim. Sci. 85:2556-2563. [DOI] [PubMed] [Google Scholar]
- 43.Ushida, K., and J. P. Jouany. 1996. Methane production associated with rumen-ciliated protozoa and its effect on protozoan activity. Lett. Appl. Microbiol. 23:129-132. [DOI] [PubMed] [Google Scholar]
- 44.Vermorel, M. 1995. Emissions annuelles de méthane d'origine digestive par les bovins en France. Variations selon le type d'animal et le niveau de production. INRA Prod. Anim. 8:265-272. [Google Scholar]
- 45.Williams, A. G., G. Fonty, and K. N. Joblin. 1994. Interactions between the rumen chytrid fungi and other microorganisms, p. 191-227. In D. O. Mountfort and C. G. Orpin (ed.), The anaerobic fungi. Marcel Dekker, New York, NY.
- 46.Wolin, M. J., T. L. Miller, and C. S. Stewart. 1997. Microbe-microbe interactions, p. 467-488. In P. N. Hobson and C. S. Stewart (ed.), The rumen microbial ecosystem. Chapman and Hall, London, United Kingdom.
- 47.Xu, K., H. Liu, G. Du, and J. Chen. 2009. Real-time PCR assays targeting formyltetrahydrofolate synthetase gene to enumerate acetogens in natural and engineered environments. Anaerobe 15:204-213. [DOI] [PubMed] [Google Scholar]