Fig. 4.
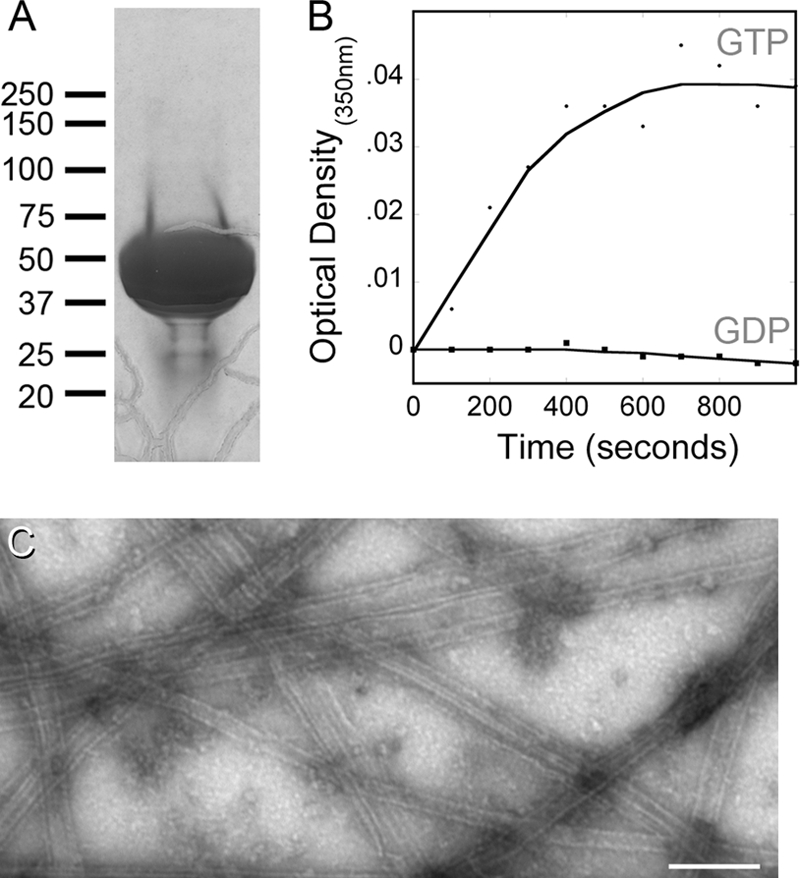
Purified tubulin is free of discernible MAPs and shows GTP-dependent assembly. (A) An overloaded Coomassie blue-stained SDS-PAGE gel illustrates the purity of DEAE-purified, cycled L136F tubulin used for biochemistry experiments; the absence of contaminating proteins (particularly any high-molecular-weight microtubule-associated proteins) is typical of all our protein purifications. (B) Assembly of I252L tubulin (as well as the wild-type and L136F samples [data not shown]) is dependent upon the presence of GTP (circles). There was no change in optical density when the assembly reaction mixture contained GDP (squares), indicating that the light scattering reflects assembly rather than aggregation of denatured protein. (C) Electron microscopy of negatively stained samples demonstrated that tubulins polymerize to microtubules and other polymeric forms in the presence of GTP. The wild-type tubulin sample is shown, but all tubulins assembled into similar polymers in this assay. Bar, 200 μm.
