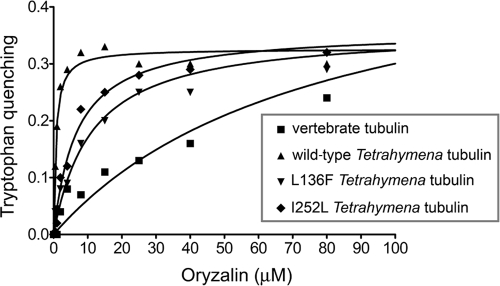
Fig. 5.
Binding curves for association of tubulin with oryzalin. Oryzalin binding was modeled using a one-site binding, nonlinear fit. Wild-type T. thermophila tubulin, L136F T. thermophila tubulin, and I252L T. thermophila tubulin all have similar maximal quench values, indicating that oryzalin interacts in the same fashion with each of the three tubulins. The binding curve and maximal quenching are different for vertebrate tubulin. These data indicate that the Kd values for oryzalin are 77 μM for vertebrate tubulin and 0.44 μM for wild-type T. thermophila tubulin, which is reduced to 11 μM for L136F T. thermophila tubulin and 6.7 μM for I252L T. thermophila tubulin.