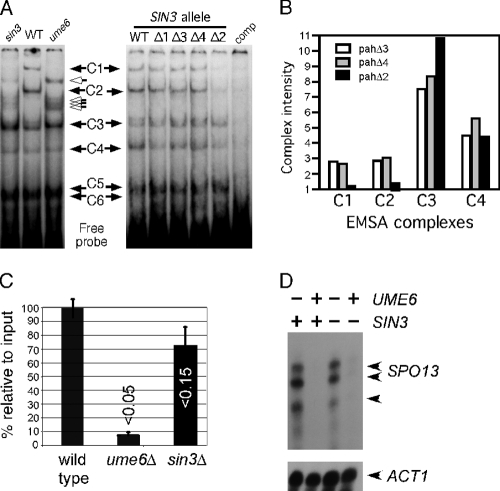
Fig. 2.
Sin3p is required for Ume6p-dependent complex formation. (A) Left, extracts prepared from RSY10 (WT), RSY305 (sin3Δ), and RSY301 (ume6Δ) were incubated with the 32P-labeled SPO13 URS1 promoter element (URS1SPO13; see Materials and Methods for details). Six protein-DNA complexes (C1 to C6) are indicated by the black arrows. The open arrows indicate new complexes that are observed in the sin3 and ume6 extracts. The C5 and C6 complexes do not resolve under these gel conditions (see Fig. 3). Right, the experiments were repeated with extracts prepared from mid-log-phase RSY278 (sin3Δ) harboring plasmids containing the wild-type SIN3 gene or the different PAH mutant derivatives as indicated. comp, 150-fold excess of unlabeled competitor URS1SPO13 double-stranded oligonucleotide added to the reaction. (B) Complex intensities of C1, C2, C3, and C4 were quantitated from pahΔ2, pahΔ3, and pahΔ4 extracts as indicated. Complex intensities are given in arbitrary units. (C) Chromatin immunoprecipitations were conducted with Ume6p antibodies in extracts prepared from three independent wild-type (RSY10), ume6Δ (RSY291), or sin3Δ (RSY579) cultures. Enrichment over no antibody control was established for each sample with the wild type set to 100%. The data are presented as the means ± standard deviations (SD) of results from three independent experiments (P values are indicated; t test). (D) S1 nuclease protection assays were conducted with total RNA prepared from wild-type (RSY273), ume6Δ (RSY272), sin3Δ (RSY275), and sin3Δ ume6Δ (RSY274) vegetative cultures. SPO13-specific bands are indicated by the arrows. ACT1 mRNA levels were used to control for the poly(A)+ percentages in the total RNA preparations.