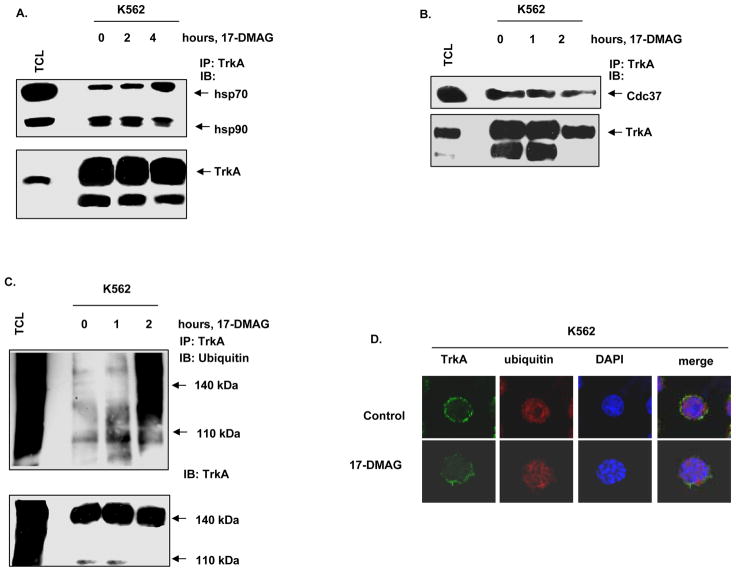
Figure 2. 17-DMAG inhibits the chaperone association of TrkA with hsp90 and promotes polyubiquitylation of TrkA.
A. K562 cells were treated with 1.0 μM of 17-DMAG for the indicated time intervals. Following this, total cell lysates were harvested, and TrkA was immunoprecipitated. The immunoprecipitates were resolved by SDS PAGE and immunoblotted for hsp70, hsp90 and TrkA. B. K562 cells were treated with 1.0 μM, 17-DMAG for the indicated exposure intervals. Following this, TrkA immunoprecipitates were resolved by SDS PAGE and immunoblotted with anti-cdc37 and anti-TrkA to assess binding of cdc37 to TrkA. C. K562 cells were treated with 1.0 μM, 17-DMAG for the indicated time intervals. Following this, TrkA was immunoprecipitated from the total cell lyastes and the immunoprecipitates were resolved by SDS PAGE. Polyubiquitylation of TrkA was determined by immunoblot analysis using an anti-ubiquitin antibody. D. K562 cells were exposed to DMSO or 0.25 μM, 17-DMAG for 24 hours. Cells were then fixed, permeabilized and immunostained for TrkA (green) and Ubiquitin (red). Nuclei were stained with DAPI. The slides were imaged with a LSM 510 meta confocal microscope using a 63×/1.2W lens.