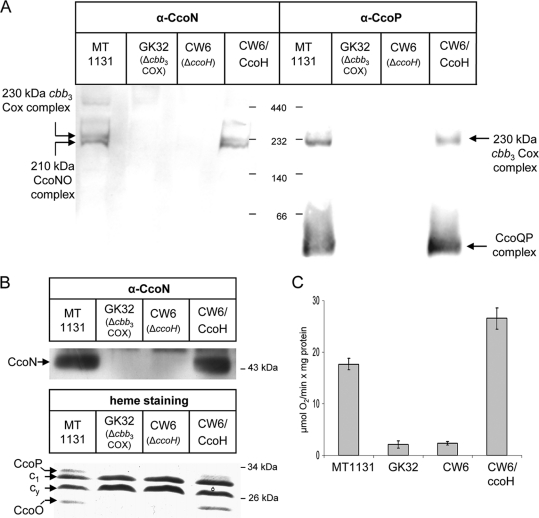
FIG. 1.
CcoH is essential for cbb3-Cox stability and activity in R. capsulatus. (A) Intracytoplasmic membranes (ICMs; 50 μg of protein) of the indicated R. capsulatus strains were solubilized with dodecylmaltoside and separated by BN-PAGE. CW6/CcoH corresponds to a genomic ccoH deletion mutant carrying a copy of ccoH on a plasmid. After being transferred onto a polyvinylidene difluoride (PVDF) membrane, complexes were decorated with antibodies against the CcoN and CcoP subunits (α-CcoN and α-CcoP). (B) The ICMs shown in panel A were separated on Tris-tricine gels and either transferred onto PVDF membranes to detect the steady-state amount of CcoN (upper panel) or analyzed by heme staining, which revealed the membrane-bound c-type cytochromes of R. capsulatus (lower panel). Indicated are the subunits CcoP and CcoO of cbb3-Cox, cyt c1 of the bc1 complex, and cyt cy. (C) cbb3-Cox activities in different ICMs were measured as oxygen uptake activities in at least three independent experiments.