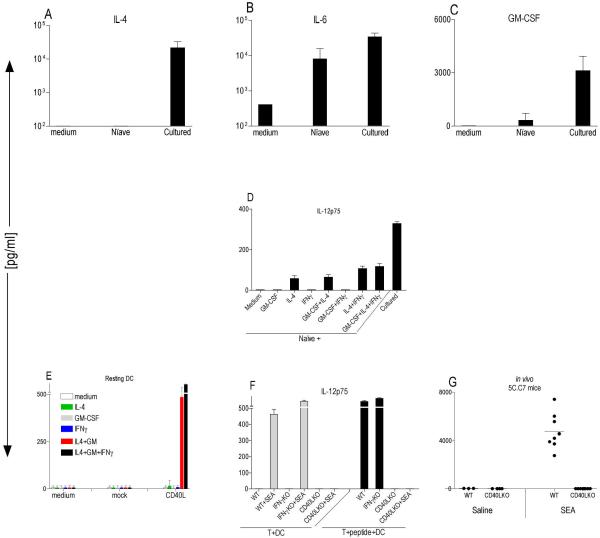
Figure 3. A combination of CD40L, IL-4 and GM-CSF will induce IL-12p75 production.
(A–C) B10.A-RAG2KO BM-DCs were preactivated with 200ng/ml LPS for 24h and washed before coculture at 2×104 cells/well with 1×105 naïve or antigen-activated 5C.C7 T cells +0.1μM MCC peptide in a 96 well U-bottom plate. After 48hrs, various cytokines were measured in the CSN using multiplex cytokine array (SearchLight). These data are expressed as the mean ± SD composite of three independent experiments. (D) BMDCs from B10.A-RAG2KO mice were preactivated with 200ng/ml LPS for 25hr then washed and cocultured at 2×104 cells/well with 2×105 sorted naïve or cultured 5C.C7 T [Tg] cells ±0.1μM MCC peptide in the presence or absence of 100ng/ml of various cytokines. CSN were tested for the presence of IL-12p75 with specific ELISA (R&D System) (E) Resting BM-DCs from B10.A-RAG2KO mice cocultured at 2×105 cells/well with 5×105 cell/well NIH-3T3 mock or NIH-3T3 CD40L expressing cells. Various cytokines were added to the coculture at final concentration of 100ng/ml in a 48-well plate. CSN weretested after 48hr coculture for the presence of IL-12p75 with specific ELISA (R&D System). These data are expressed as the mean ± SD composite of three independent experiments. (F) BM-DCs from B10.A-Rag2KO mice were preactivated with 100ng/ml LPS for 27hr then washed and cocultured at 2×104 cells/well with 2×105 WT, IFN-γKO and CD40L 5C.C7 T [Tg] cells ± 0.1μM MCC peptide and ±5μg/ml SEA in a 96 well U-bottom plate for 48 hr. CSN were tested for the presence of IL-12p75 with specific ELISA (R&D System). These data are expressed as the mean ± SD triplicates from a 96-well plate. (G) WT 5C.C7 TCR [Tg] (n=11), or CD40LKO 5C.C7 TCR [Tg] (n=12) mice were injected intravenously with either saline or 10μg of SEA and after 6 hrs serum was tested for the presence of IL-12p75 using a multiplex cytokine array (Ray Biotech).