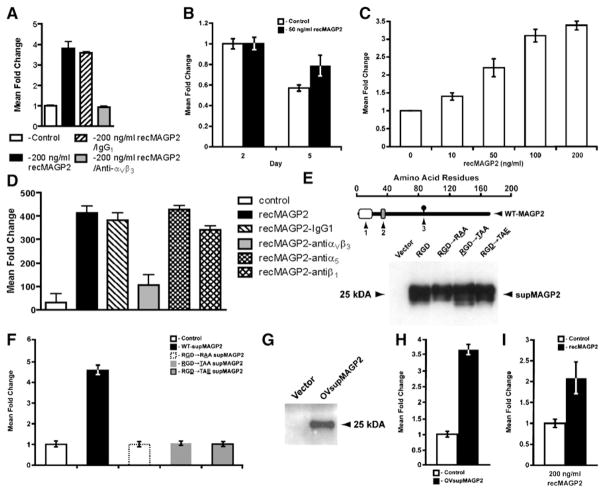
Figure 5. RecMAGP2 Stimulates Adhesion, Survival, Motility, and Invasion of HUVECs In Vitro.
(A) HUVECs cultured on recMAGP2 showed increased adhesion (p < 0.0002) when compared to anti-αVβ3 integrin antibody preincubation.
(B) Enhanced survival of HUVECs treated with recMAGP2 (p < 0.05).
(C) Dose-dependent HUVEC motility in response to recMAGP2 protein was significant at all concentrations evaluated (p ≤ 0.01).
(D) Anti-αVβ3 integrin antibody attenuated re-cMAGP2-stimulated HUVEC motility through αVβ3 receptor inhibition (p < 0.002), whereas anti-α5 and anti-β1 integrin antibody treatments did not.
(E) Successful processing and secretion of wild-type supMAGP2 and RGD motif mutant proteins by 293T cells (1, signal sequence; 2, RGD motif; 3, N-linked carbohydrate consensus sequence; the underlined letter refers to the mutated RGD motif amino acid).
(F) supMAGP2 stimulated HUVEC motility (p = 0.002), whereas the three mutant constructs had no significant effect.
(G) Proper processing and secretion of wild-type OVsupMAGP2 protein by UCI107 ovarian cancer cells.
(H) Ovarian cancer cell-derived OVsupMAGP2 induced HUVEC motility (p < 0.001).
(I) Increased invasion of recMAGP2-treated HU-VECs into matrigel, as compared to untreated cells (p < 0.05). For (A)–(D), (F), (H), and (I), the data represents the mean ± SD of three independent experiments.