Abstract
Purpose
Compare the efficacy of treatment of pneumococcal keratitis with cholesterol, moxifloxacin, or a mixture of the two (moxifloxacin/cholesterol).
Materials and Methods
New Zealand white rabbits were injected intrastromally with 106 colony-forming units (CFU) of a clinical keratitis strain of Streptococcus pneumoniae. Eyes were examined before and after treatment of topical drops every 2 hr from 25 to 47 hr post-infection (PI). Corneas were harvested to quantitate bacterial CFU, and myeloperoxidase (MPO) activity was measured at 48 hr PI. Eyes were extracted for histology. Minimal inhibitory concentrations (MICs) were determined for each compound.
Results
Eyes treated with moxifloxacin/cholesterol had a significantly lower mean slit lamp examination (SLE) score than eyes treated with phosphate-buffered saline (PBS), moxifloxacin alone, or cholesterol alone (P ≤ 0.02). A significantly lower log10 CFU was recovered from corneas treated with moxifloxacin/cholesterol and moxifloxacin alone as compared to corneas of eyes treated with PBS or cholesterol alone (P < 0.01). At 48 hr PI, significantly lower MPO activity was quantitated from eyes treated with moxifloxacin/cholesterol as compared to eyes treated with cholesterol or moxifloxacin alone (P ≤ 0.046). Eyes treated with moxifloxacin/cholesterol had fewer immune cells and less corneal destruction than eyes from all other treatment groups. The MIC for moxifloxacin alone was 0.125 µg/mL, and cholesterol alone was unable to inhibit growth at any of the concentrations tested. The MIC for moxifloxacin when combined with 1% cholesterol was 0.0625 µg/mL.
Conclusions
Treatment with a mixture of moxifloxacin and cholesterol significantly lowers the severity of infection caused by pneumococcal keratitis as compared to treatment with moxifloxacin alone, cholesterol alone, or PBS. This treatment mixture eradicates the bacteria in the cornea, unlike treatment with PBS or cholesterol alone. Using cholesterol with moxifloxacin as a treatment for bacterial keratitis could help lower the clinical severity of the infection.
Keywords: Cholesterol, Keratitis, Moxifloxacin, Streptococcus pneumoniae
INTRODUCTION
Gram-positive cocci are responsible for about 67% of contact lens-related bacterial keratitis cases. 1 Streptococcus pneumoniae is one of the gram-positive bacteria commonly isolated from the cornea in bacterial keratitis infections.2–6 Treatment of bacterial keratitis is important for visual outcome. Monotherapy with a fluoroquinolone, such as moxifloxacin, is commonly used to treat bacterial keratitis.7 Both gram-positive and gram-negative bacteria have been shown to consistently have a lower resistance to moxifloxacin than numerous other drugs.8
Pneumolysin, a hemolysin produced by S. pneumoniae, is a major virulence factor in both ocular and non-ocular infections. Johnson et al.9 observed that a pneumolysin-deficient mutant of S. pneumoniae caused a less severe keratitis infection than the parent strain. This toxin belongs to a family of cholesterol-dependent cytolysins (CDCs) that form pores in host cell membranes.10 It has long been known that treatment of pneumolysin with exogenous cholesterol inhibits the activity of this toxin.11 A previous study reported that cholesterol treatment of keratitis caused by WU2, a type 3 strain of S. pneumoniae originally obtained by passage of a human isolate in mice,12 resulted in a lower severity of infection as compared to treatment with phosphate-buffered saline (PBS).13 Treatment with cholesterol decreased the amount of bacteria in vivo (corneal infection) and in vitro (minimal inhibitory concentration [MIC] assay). Previous reports of the ability of exogenous cholesterol to inhibit pneumolysin were primarily performed in vitro, such as in human monocytes14 and lymphocytes,15 neuroblastoma cells,16,17 fibroblasts, and astrocytes.17 One in vivo study of note examined the ability of a variety of purified CDCs to kill mice.18 The authors showed that pneumolysin and the other CDCs tested were able to kill mice and that lethality was directly proportional to the hemolytic activity of each toxin. Addition of exogenous cholesterol to one of the CDCs (listeriolysin O) completely abrogated the ability of the toxin to kill mice. Although pneumolysin was not specifically tested in the inhibition experiment, the results indicated that similar inhibition by cholesterol would be observed for any of the CDCs.18 To date, no studies have reported the efficacy of antibiotic/cholesterol combination treatment of pneumococcal keratitis caused by a clinical ocular strain. Therefore, the experiments described herein aimed to determine whether a mixture of moxifloxacin and cholesterol (moxifloxacin/cholesterol) would be more efficacious in treatment of pneumococcal keratitis as compared to treatment with either cholesterol or moxifloxacin alone.
METHODS
Bacterial Growth
S. pneumoniae K1263, a type 35f clinical keratitis strain, was kindly provided by Regis Kowalski (Charles T. Campbell Eye Microbiology Lab, Pittsburgh, Pennsylvania, USA). Bacterial colonies were isolated on 5% sheep blood agar and incubated overnight at 37°C and 5% CO2. Todd Hewitt broth containing 0.5% yeast extract (THY) was inoculated with one colony and incubated at 37°C in 5% CO2 overnight. The overnight culture was inoculated into fresh THY at a 1:100 dilution. The bacteria were grown to an optical density (OD) at A600 that corresponded to approximately 108 colony-forming units (CFU) per ml. Accuracy of the bacterial CFU was verified by colony counts of serial dilutions.
Infection
New Zealand white rabbits (Harlan Sprague Dawley, Inc., Oxford, Michigan, USA) were used in these studies and maintained according to the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. Each rabbit was anesthetized by an intramuscular injection of a mixture of ketamine hydrochloride (100 mg/ ml; Butler Company, Columbus, Ohio, USA) and xylazine (100 mg/ml; Butler Company). Proparacaine hydrochloride drops were applied to each eye before injection. Bacterial cultures were diluted such that each cornea was infected with approximately 106 CFU in a volume of 10 µl. A 30-gauge needle was used to inoculate the bacteria into the stroma of each eye. The use of animals in this research complied with the guidelines of, and was approved by, the Institutional Animal Care and Use Committee of the University of Mississippi Medical Center.
Slit Lamp Examination (SLE)
SLE was performed before and after treatment. Seven parameters were used for determining the severity of infection: injection, chemosis, iritis, fibrin, hypopyon, corneal infiltrate, and corneal inflammation.9 Each parameter was given a grade from 0 (no pathogenesis) to 4 (maximal pathogenesis), resulting in a total score with a theoretical maximum of 28. Each eye was scored by two examiners who were blind to the treatment groups, and the two scores were averaged.
Treatment Regimen
Treatment commenced at 25 hr post-infection (PI). The rabbits were randomized into four treatment groups by an investigator that was not involved in the examination and scoring. The treatment groups were sterile PBS mixed with PBS containing 20% glycerol (1:1 volume:volume; “PBS”), PBS mixed with 1% soluble cholesterol (Sigma-Aldrich, St. Louis, Missouri, USA) in PBS containing 20% glycerol (1:1; “cholesterol”), moxifloxacin (Vigamox®, 5 mg/ml; Alcon, Fort Worth, Texas, USA) mixed with PBS containing 20% glycerol (1:1; “moxifloxacin”), and moxifloxacin mixed with 1% soluble cholesterol in PBS containing 20% glycerol (1:1; “moxifloxacin/cholesterol”). Two drops were placed in each eye every 2 hr for a total of 12 doses (n = 16 eyes for each treatment group; Table 1).
TABLE 1.
Treatment regimen
| Treatment group | Composition | n |
|---|---|---|
| PBS | PBS + PBS containing 20% glycerol (1:1 vol:vol) | 16 |
| Cholesterol | PBS + 1% cholesterol in PBS containing 20% glycerol (1:1) | 16 |
| Moxifloxacin | Moxifloxacin + PBS containing 20% glycerol (1:1) | 16 |
| Moxifloxacin/Cholesterol | Moxifloxacin + 1% cholesterol in PBS containing 20% glycerol (1:1) | 16 |
Each eye was treated every 2 hr for 12 doses starting at 25 hr PI.
CFU Recovery
Rabbits were euthanized with an intravenous overdose of sodium pentobarbital (100 mg/kg; Sigma-Aldrich) at 24 hr PI (for quantitation of baseline CFU per cornea) or at 48 hr PI (after the treatments and final examination). Corneas were harvested, homogenized, serially diluted in sterile PBS, and plated in triplicate on blood agar. Plates were incubated overnight at 37°C, and colonies were counted.
Myeloperoxidase (MPO) Activity Assay
The MPO activity of polymorphonuclear cells (PMNs) in infected corneas was determined using a colorimetric assay as described previously.19 Purified MPO (Sigma-Aldrich) served as a positive control. MPO activity was expressed as MPO units.
Minimal Inhibitory Concentration (MIC) Assays
The MICs of cholesterol in PBS containing 20% glycerol, moxifloxacin, and a mixture of 1% cholesterol in 20% PBS with serially diluted moxifloxacin against K1263 were determined using the macrodilution broth method according to the Clinical and Laboratory Standards Institute.20 Each dilution was plated in triplicate, incubated, and counted because observation of turbidity was previously determined to not be a reliable determination when cholesterol was used in the assay.13 The MIC for each test was determined to be the lowest concentration at which no CFUs were observed, taking into account the final two-fold dilution of each antibiotic when the bacterial suspension was added. Two independent assays were performed and yielded the same results.
Histopathology
Whole eyes were removed at 48 hr PI and histopathology was performed by Excalibur Pathology, Inc. (Moore, Oklahoma, USA) using hematoxylin and eosin staining.
Statistics
Data were analyzed using the Statistical Analysis System (SAS) program for computers (Cary, North Carolina, USA). Clinical SLE scores were analyzed using a non-parametric one-way analysis of variance. Bacterial CFU were analyzed using the General Linear Models Procedure of Least Squares Means. All experiments were performed twice, yielding similar results, and the data from the two experiments were combined. Statistical analysis of the MPO data was performed using a Student’s t-test. P < 0.05 was considered significant.
RESULTS
Rabbit Keratitis Model
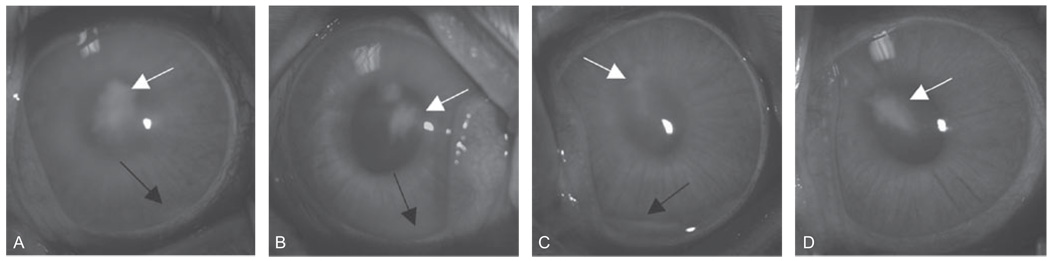
Eyes were examined prior to treatment to demonstrate that clinical severity was equivalent among all of the groups. Twenty-four hours PI (pre-treatment), the SLE scores were similar for the PBS, cholesterol, moxifloxacin, and moxifloxacin/cholesterol treatment groups (P ≥ 0.43; Table 2). Forty-eight hours PI (post-treatment), eyes treated with moxifloxacin/cholesterol had a significantly lower mean SLE score than eyes treated with PBS, cholesterol, or moxifloxacin alone (P ≤ 0.016). There was no significant difference in clinical severity among any of the other treatment groups (P ≥ 0.12; Table 2, Figure 1).
TABLE 2.
Mean clinical scores* representing keratitis severity 24 hr and 48 hr PI
| Treatment | n | SLE 24 hr PI (pre-treatment) |
SLE 48 hr PI (post-treatment) |
|---|---|---|---|
| PBS | 16 | 4.93 ± 0.29 | 8.55 ± 0.67 |
| Cholesterol | 16 | 4.60 ± 0.48 | 7.45 ± 0.95 |
| Moxifloxacin | 16 | 4.68 ± 0.17 | 7.24 ± 0.44 |
| Moxifloxacin/ Cholesterol** |
16 | 4.63 ± 0.25 | 4.41 ± 0.71 |
SLE = Slit lamp examination.
± Standard errors of the means.
SLE score 24 hr PI is significantly lower than PBS-, cholesterol-, and moxifloxacin-treated eyes.
FIGURE 1.
Representative eyes 48 hr PI. Eyes were treated with PBS (A), cholesterol (B), moxifloxacin (C), or moxifloxacin/cholesterol (D). Haziness and hypopyon (black arrow) were observed in eyes treated with PBS, cholesterol, or moxifloxacin. Infiltrate (white arrow) at the site of injection was observed in eyes from all treatment groups.
Corneal CFU Recovery
The mean log10 CFU ± standard error of the mean (SEM) of S. pneumoniae recovered from corneas 24 hr PI (pre-treatment) was 6.64 ± 0.49 (Table 3). Forty-eight hours PI (post-treatment), moxifloxacin-treated and moxifloxacin/cholesterol-treated eyes had significantly lower log10 CFU recovered from corneas than corneas treated with PBS or cholesterol alone (P < 0.0001). Moxifloxacin and moxifloxacin/cholesterol were equally efficient at sterilizing the corneas (P = 1.00).
TABLE 3.
Log10 CFU recovered from the cornea 24 hr and 48 hr PI
| Test | n | Average Log10 CFU counts ± SEM |
|---|---|---|
| Baseline at 24 hr | 10 | 6.64 ± 0.49 |
| PBS* | 11 | 4.26 ± 0.51 |
| Cholesterol* | 11 | 3.77 ± 0.44 |
| Moxifloxacin*’** | 12 | 0.00 ± 0.00 |
| Moxifloxacin/ Cholesterol*’** |
12 | 0.00 ± 0.00 |
Data determined at 48 hours PI.
Significantly lower than PBS- and cholesterol-treated corneas.
MPO Assay
Mean MPO units, which indicate PMN activity, were determined pre- and post-treatment. Moxifloxacin/cholesterol treatment was effective at reducing the baseline MPO units (P = 0.006). Moreover, corneas treated with moxifloxacin/cholesterol had significantly lower MPO activity than corneas treated with cholesterol alone or moxifloxacin alone (P ≤ 0.046). No significant difference was observed between any of the other treatment groups (P ≥ 0.35; Table 4).
TABLE 4.
MPO units recovered from corneas infected with S. pneumoniae
| Test | n | Average MPO units ± SEM |
|---|---|---|
| Baseline at 24 hr | 12 | 5.79 ± 0.73 |
| PBS | 12 | 4.45 ± 2.08 |
| Cholesterol | 12 | 6.03 ± 1.84 |
| Moxifloxacin | 12 | 4.68 ± 0.89 |
| Moxifloxacin/ Cholesterol* |
12 | 2.95 ± 0.73 |
Significantly lower than cholesterol- and moxifloxacin-treated corneas.
MICs
The MIC for moxifloxacin alone was 0.125 µg/mL, and cholesterol alone was unable to inihibit growth at any of the concentrations tested. The MIC for moxifloxacin when combined with 1% cholesterol was 0.0625 µg/mL.
Histopathology
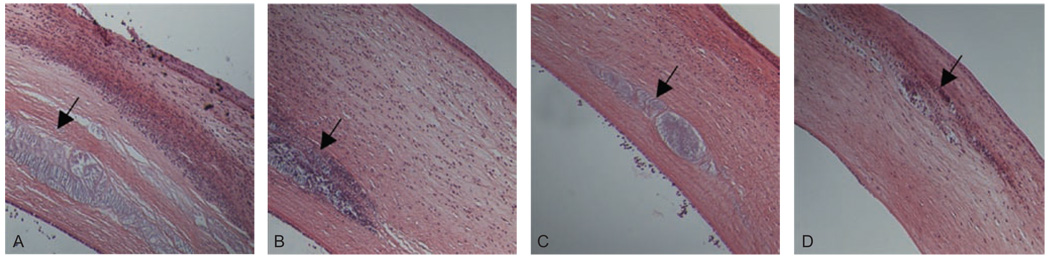
All eyes were removed in whole, sectioned, and stained with hematoxylin and eosin. More immune cells were observed in the corneas of eyes treated with PBS, cholesterol alone, or moxifloxacin alone as compared to eyes treated with the moxifloxacin/cholesterol mixture (Figure 2). Injection sites were observed in all treatment groups (Figure 2; black arrows).
FIGURE 2.
Representative histology of infected eyes at 48 hr PI. Eyes were treated with PBS (A), cholesterol (B), moxifloxacin (C), or moxifloxacin/cholesterol (D). Fewer immune cells were observed in moxifloxacin/cholesterol-treated eyes as compared to the other treatment groups. Arrow indicates injection site.
DISCUSSION
The current findings indicate that combination therapy with moxifloxacin and soluble cholesterol would be an effective means of not only killing S. pneumoniae in the cornea, but also reducing the clinical severity of pneumococcal keratitis. A study previously published by this laboratory showed that treatment of pneumococcal keratitis caused by WU2 (a non-ocular strain) with cholesterol alone could significantly lower the severity of pneumococcal keratitis infection as well as the bacterial load.13 The findings of the current study were different from the previous study in that cholesterol alone was unable to improve the clinical scores or significantly reduce the bacterial load. Several possible reasons could account for the contrasting results. Different strains were used for the two studies, one of which was a non-ocular strain and another of which was an ocular strain. The ocular strain used in the current study could be more resistant to treatment with cholesterol, which highlights the importance of using combination therapy with an effective antibiotic.
Another reason for the difference in findings between the previous study and the current study could be the difference in inoculum dose. The previous study used an inoculum of 105 CFU, whereas the current study used an inoculum of 106 CFU. The intended inoculum for the current study was 105 CFU and the bacteria were cultured to an optical density (OD) that was anticipated to contain 105 CFU per 10 µl; however, quantitation of the inoculum by plating of serial dilutions showed that the actual inoculum was 106 CFU. Supporting this idea was that about 1 log10 unit more bacteria were recovered from cholesterol-treated corneas 48 hr PI in the current study (3.77 ± 0.44) than those in the previous study (2.64 ± 0.50).13 This finding also supports the importance of using combination therapy with an antibiotic so that the bacteria can be completely killed instead of only reduced. Also, treatment with moxifloxacin/ cholesterol significantly lowered the MPO activity compared to eyes treated with moxifloxacin or cholesterol alone (Table 4) which is in agreement with fewer PMNs detected by histology (Figure 2) and the significantly fewer log10 CFU recovered from the corneas, as compared to PBS or cholesterol-treated eyes. The MICs against the bacteria support these data as well. In vitro bacteria were not susceptible to cholesterol, which was also observed in vivo. Bacteria were susceptible to moxifloxacin and moxifloxacin/cholesterol in vivo and in vitro. Even though cholesterol alone did not inhibit growth of bacteria, cholesterol appeared to have a synergistic effect when mixed with moxifloxacin as demonstrated by the lower MIC when compared to moxifloxacin alone. For the previous study, cholesterol alone had a MIC of 1% and lowered the clinical severity of keratitis caused by strain WU2.13 For the present study, cholesterol alone had no MIC and did not lower the clinical severity of the clinical isolate used for the keratitis infection. The differences between the results of the two studies are probably due to the differences in the two strains used.
Thirdly, the amount of pneumolysin activity could likely be involved in differences between strains, as pneumolysin has been shown to be a major virulence factor in pneumococcal keratitis9 and cholesterol is the host substrate for pneumolysin.10 A hemolysis assay was performed in the current study using WU2 as previously described,21 and determined that the pneumolysin activity of WU2 was slightly lower (83% hemolysis) compared to the clinical keratitis strain used for this study (89% hemolysis). A recent report showed that pneumolysin is a membrane-bound toxin, and depending on serotype, pneumolysin could be found predominantly in cell wall fractions or protoplast fractions of S. pneumonia.22 Perhaps WU2 (a type 3 strain) contains a higher amount of pneumolysin in the cell wall than K1263 (a type 35f strain), which would allow the pneumolysin to be more accessible to outside agents such as exogenously added cholesterol.
Moxifloxacin is commonly used to treat bacterial keratitis due to its broad range of bacterial susceptibility.7,8 It does not, however, lower the clinical severity caused by pneumococcal keratitis. This study showed that treatment of S. pneumoniae keratitis with a mixture of cholesterol and moxifloxacin both sterilizes the cornea and significantly lowers the severity of infection compared to treatment with PBS, cholesterol alone, or moxifloxacin alone. This combination therapy appears to provide a double benefit in that the antibiotic effectively kills the bacteria, and the cholesterol inhibits the toxic effects of pneumolysin, which include host cell lysis and/or activation of complement that induces a deleterious and damaging host immune response in the eye. Treatment with this mixture could lessen the severity of visual outcome caused by pneumococcal infection in humans.
ACKNOWLEDGMENT
The authors wish to acknowledge financial support from the National Eye Institute, National Institutes of Health (Public Health Services Grant R01EY016195).
Footnotes
Declaration of interest: The authors report no conflict of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- 1.Bourcier T, Thomas F, Borderie V, et al. Bacterial keratitis: Predisposing factors, clinical and microbiological review of 300 cases. Br J Ophthalmol. 2003;87:834–838. doi: 10.1136/bjo.87.7.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong T, Ormonde S, Gamble G, et al. Severe infective keratitis leading to hospital admission in New Zealand. Br J Ophthalmol. 2003;87:1103–1108. doi: 10.1136/bjo.87.9.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bharathi M, Ramakrishnan R, Vasu S, et al. In-vitro efficacy of antibacterials against bacterial isolates from corneal ulcers. Indian J Ophthalmol. 2002;50:109–114. [PubMed] [Google Scholar]
- 4.Donnenfeld E, O’Brien T, Solomon R, et al. Infectious keratitis after photorefractive keratectomy. Ophthalmology. 2003;110:743–747. doi: 10.1016/S0161-6420(02)01936-X. [DOI] [PubMed] [Google Scholar]
- 5.Parmar P, Salman A, Kalavathy C, et al. Pneumococcal keratitis: A clinical profile. Clin Experiment Ophthalmol. 2003;31:44–47. doi: 10.1046/j.1442-9071.2003.00598.x. [DOI] [PubMed] [Google Scholar]
- 6.Varaprasathan G, Miller K, Lietman T, et al. Trends in the etiology of infectious corneal ulcers at the F. I. Proctor Foundation. Cornea. 2004;23:360–364. doi: 10.1097/00003226-200405000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Allan BD, Dart JK. Strategies for the management of microbial keratitis. Br J Ophthalmol. 1995;79:777–786. doi: 10.1136/bjo.79.8.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sueke H, Kaye S, Neal T, et al. Minimum inhibitory concentrations of standard and novel antimicrobials for isolates from bacterial keratitis. Invest Ophthalmol Vis Sci. 2010;51:2519–2524. doi: 10.1167/iovs.09-4638. [DOI] [PubMed] [Google Scholar]
- 9.Johnson M, Hobden J, Hagenah M, et al. The role of pneumolysin in ocular infections with Streptococcus pneumoniae. Curr Eye Res. 1990;9:1107–1114. doi: 10.3109/02713689008997584. [DOI] [PubMed] [Google Scholar]
- 10.Tweten RK. Cholesterol-dependent cytolysins, a family of versatile pore-forming toxins. Infect Immun. 2005;73:6199–6209. doi: 10.1128/IAI.73.10.6199-6209.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shumway CN, Klebanoff SJ. Purification of pneumolysin. Infect Immun. 1971;4:388–392. doi: 10.1128/iai.4.4.388-392.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Briles DE, Nahm M, Schroer K, et al. Antiphosphocholine antibodies found in normal mouse serum are protective against intravenous infection with type 3 Streptococcus pneumoniae. J Exp Med. 1981;153:694–705. doi: 10.1084/jem.153.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marquart ME, Monds KS, McCormick CC, et al. Cholesterol as treatment for pneumococcal keratitis: Cholesterol-specific inhibition of pneumolysin in the cornea. Invest Ophthalmol Vis Sci. 2007;48:2661–2666. doi: 10.1167/iovs.07-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nandoskar M, Ferrante A, Bates E, et al. Inhibition of human monocyte respiratory burst, degranulation, phospholipid methylation and bactericidal activity by pneumolysin. Immunology. 1986;59:515–520. [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrante A, Rowan-Kelly B, Paton JC. Inhibition of in vitro human lymphocyte response by the pneumococcal toxin pneumolysin. Infect Immun. 1984;46:585–589. doi: 10.1128/iai.46.2.585-589.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iliev AI, Djannatian JR, Nau R, et al. Cholesterol-dependent actin remodeling via RhoA and Rac1 activation by the Streptococcus pneumoniae toxin pneumolysin. Proc Natl Acad Sci U S A. 2007;104:2897–2902. doi: 10.1073/pnas.0608213104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iliev A, Djannatian J, Opazo F, et al. Rapid microtubule bundling and stabilization by the Streptococcus pneumoniae neurotoxin pneumolysin in a cholesterol-dependent, non-lytic and Src-kinase dependent manner inhibits intracellular trafficking. Mol Microbiol. 2009;71:461–477. doi: 10.1111/j.1365-2958.2008.06538.x. [DOI] [PubMed] [Google Scholar]
- 18.Watanabe I, Nomura T, Tominaga T, et al. Dependence of the lethal effect of pore-forming haemolysins of Gram-positive bacteria on cytolytic activity. J Med Microbiol. 2006;55:505–510. doi: 10.1099/jmm.0.46333-0. [DOI] [PubMed] [Google Scholar]
- 19.Hobden JA, Hill JM, Engel LS, et al. Age and therapeutic outcome of experimental Pseudomonas aeruginosa keratitis treated with ciprofloxacin, prednisolone, and flurbiprofen. Antimicrob Agents Chemother. 1993;37:1856–1859. doi: 10.1128/aac.37.9.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; approved standard, seventh edition. Clinical and Laboratory Standards Institute document M7-A7. Wayne, Pennsylvania: Clinical and Laboratory Standards Institute; 2006. [Google Scholar]
- 21.Sanders M, Norcross E, Moore Q, et al. A comparison of pneumolysin activity and concentration in vitro and in vivo in a rabbit endophthalmitis model. Clin Ophthalmol. 2008;2:793–800. doi: 10.2147/opth.s3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Price KE, Camilli A. Pneumolysin localizes to the cell wall of Streptococcus pneumoniae. J Bacteriol. 2009;191:2163–2168. doi: 10.1128/JB.01489-08. [DOI] [PMC free article] [PubMed] [Google Scholar]