Abstract
Rather than being a static, species specific trait, reproductive behavior in female amphibians is variable within an individual during the breeding season when females are capable of reproductive activity. Changes in receptivity coincide with changes in circulating estrogen. Estrogen is highest at the point when females are ready to choose a male and lay eggs. At this time female receptivity (her probability of responding to a male vocal signal) is highest and her selectivity among conspecific calls (measured by her probability of responding to a degraded or otherwise usually unattractive male signal) is lowest. These changes occur even though females retain the ability to discriminate different acoustic characteristics of various conspecific calls. After releasing her eggs, female amphibians quickly become less receptive and more choosy in terms of their responses to male sexual advertisement signals. Male vocal signals stimulate both behavior and estrogen changes in amphibian females making mating more probable. The changes in female reproductive behavior are the same as those generally accepted as indicative of a change in female sexual arousal leading to copulation. They are situationally triggered, gated by interactions with males, and decline with the consummation of sexual reproduction with a chosen male. The changes can be triggered by either internal physiological state or by the presence of stimuli presented by males, and the same stimuli change both behavior and physiological (endocrine) state in such a way as to make acceptance of a male more likely. Thus amphibian females demonstrate many of the same general characteristics of changing female sexual state that in mammals indicate sexual arousal.
Keywords: amphibians, reproduction, female, communication, estrogen
Introduction
Approaching a comparative analysis of female sexual arousal seems at first a daunting task. Given the diversity of sociosexual behaviors across vertebrate species it may seem as though such comparisons are impossible, especially after considering the complicated cognitive-emotional-physiological processes involved and the diverse sensory stimuli that trigger a sexually aroused state. Yet if one looks at the general components of female sexual arousal there are processes that carry through to the sociosexual responses in many vertebrates. From the most basic perspective, female sexual arousal refers to a relatively short-term increase in female sexual motivation within the period of her overall seasonal or cyclic receptivity, that is, an increase in a female’s willingness to engage in copulatory behavior (or its equivalent) with a male. Sexual arousal is further defined as a change that is limited by a copulatory act: in analogy to what is generally understood for human sexual arousal, there should be a rapid decrease in the aroused state once the sexual interaction has been consummated. This circumscribed change indicates a motivational shift, and although in most animals this cannot be assessed directly, it should be reflected in a female’s behavior, particularly in her response to male sexual signals. If this is the essence of female sexual arousal (acknowledging that many more, and more complicated, events may be connected to this), then studying the biology of female sexual arousal in many different animal taxa becomes a tractable problem.
In addition to the episodic nature of the change (an increase in receptivity leading to sexual interaction with a male, followed by a rapid decline following the interaction) two other things characterize female sexual arousal. First, the state of arousal should be modulated by sensory signals. Although in humans a large variety of stimuli can induce a state of sexual arousal in both females and males, during the natural behavior of nonhuman vertebrates the signals that induce arousal are the signals generated by conspecific males. That is, female sexual arousal in nonhuman animals can be said to occur when an increase in receptivity is stimulated by conspecific male signals. Second, female sexual arousal should have physiological and endocrinological correlates, and the signals inducing the aroused state should modulate both the behavioral/motivational response and a physiological/endocrinological response. The nature of those conjoined responses could naturally be quite different in different species. Behavioral endocrinologists have often found, however, that common neural and physiological mechanisms underlie classes of social behavior across many vertebrate taxa. A valuable contribution of a comparative analysis of complex social and emotional behaviors such as female arousal is that they can highlight fundamental mechanisms underlying them across human and nonhuman animals.
Here we consider female sexual arousal in amphibians, emphasizing variation in female receptivity in anurans. Amphibian communication and reproductive behaviors have been studied extensively in the complementary fields of neuroethology, behavioral endocrinology, and evolutionary animal behavior. Intraspecific variation in male signals and its effect on female mate choice has been a topic of considerable attention, and work in this area has provided new insights into sexual selection and the evolution of animal signals. Much of this work has conceptualized female responses as seasonally gated but relatively fixed in their characteristics, with the goal of determining species specific patterns and intraspecific variation. Recently, several studies in amphibians and other vertebrates have shown that female receptivity and mate choice decisions are not static even over a breeding cycle. This work, on which we will focus in this review, provides the basis for examining the problem of sexual arousal in this group of vertebrates.
Sexual behaviors in female amphibians
The vertebrate class Amphibia includes three orders, Anura, Caudata (or Urodela) and Gymnophonia. Two of these orders, Anurans (i.e. frogs and toads) and Urodeles (i.e. salamanders and newts), have become useful systems in which to examine female reproductive behavior and its relationship to sex steroid and peptide hormones. Anurans and urodeles exhibit clearly defined and easily measured sexual behaviors, which has led to their popularity as model systems. There is currently so little research on social behavior in the Gymnophones that we will consider here only the other two amphibian orders.
Reproductive social behavior in anurans is organized around the species typical vocal signals produced by males. Once of the most common means by which female anurans express sexual behavior is by moving toward males producing these mate attraction signals. This behavior is termed phonotaxis (Wells, 1977). Female anurans readily exhibit this response when playbacks of conspecific male calls are presented through speakers (Ryan, 1980, 1985, 1997; Gerhardt and Huber, 2002). The phonotatic response of a female anuran is generally selective for conspecific calls but can at times include responses to calls of closely related species (Ryan et al. 2003; Lynch et al. 2005 ; Lynch et al., 2006 ; Bee, 2008). Female anurans do, however, exhibit a remarkable ability to discriminate and selectively respond to conspecific signals that contain subtle acoustic differences from other conspecific signals (Ryan, 1980; Gerhardt and Huber, 2002) a behavior which indicates a robust ability to use these differences to guide mate choice. Females in some female anuran species, such as the African clawed frog (Xenopus laevis) also vocalize to signal reproductive state (Tobias et al., 1998; Elliot and Kelley, 2007). Xenopus females produce a —rapping call to signal sexual receptivity and a —ticking call to signal an unreceptive state (Tobias et al., 1998; Elliot and Darcy, 2007). Vocalizations signaling an unreceptive state are also referred to as release calls in some anurans (Tobias et al., 1998; Boyd, 1992 ; Kelley, 1982; Diakow, 1978). Females use other behaviors to control male access. Receptive female anurans allow males to clasp them from the dorsal side, a behavior referred to as amplexus. Behaviors that hinder amplexus from occurring, such as leg extensions, are inhibited in receptive female anurans (Diakow, 1978). In short, even within a breeding season, when females are in principle ready to mate, female anurans can be receptive or unreceptive and can be selective in their sexual behavior toward particular males.
Because urodeles such as newts, particularly the rough-skinned newt (Taricha granulosa) and the red-bellied newt (Cynops pyrrhogaster), court and mate readily in the laboratory, they have also become a popular model in which to investigate the relationship between sexual behavior and reproductive physiology. In urodeles, sociosexual behavior is mediated through visual and chemical signaling rather than vocalizations. Although the details of sexually receptive behavior vary among urodeles, there is for the most part a consistent behavioral pattern in t he receptive behavior of female newts. Here female reproductive behaviors are composed of four general stages. First, the female must migrate to water where reproduction occurs. Once in the water, the female will secrete an attractant-like substance into the water. When a male responds to these substances he must perform a suite of courtship behaviors to influence the female to capture his spermatophore. As in anurans, male newts will amplex the female from the dorsal side. If unreceptive, the female will arch her back and move her head down. She will express receptivity by raising her head, allowing the male to apply pheromones. If he is successful in his courtship behaviors, the female will follow the male to the location of his spermatophore deposition and capture his spermatophore through her cloaca in order for internal fertilization to occur (Kikuyama et al. 2009 for review). Finally, the female urodele will deposit her fertilized eggs on aquatic vegetation. As in anurans, urodele females can show receptive or unreceptive behavior toward a particular male, and can transition from rejecting to accepting the sexual advances of a single male as he directs the ritualistic courtship approaches towards her.
Variation in female reproductive responses suggest variation in sexual arousal
Phonotaxis behavioral tests are the most common method for measuring female anuran reproductive behavior. The basic design is simple: females are placed in a test chamber some distance from a speaker or speakers broadcasting test stimuli. In such a situation, reproductively active females will orient toward and approach a speaker broadcasting a male call that is attractive to her. Phonotaxis tests can be employed to assay a female’s receptivity toward males simply by ascertaining whether or she will approach a conspecific call. The phonotaxis test can also be designed to assay the selectivity of the females mate choices by providing her with a variety of calls. Very selective or choosy females will respond to a narrow range of mate signals that are acceptable to them whereas less selective females will respond to a wider range of signals. Phonotaxis tests can test a female’s ability to discriminate among calls by providing different stimuli from speakers in different locations relative to her starting point. These three different types of measures can be difficult to differentiate – for example, it can be difficult to disassociate changes in selectivity from changes in receptivity (Jennions and Petrie, 1997, for review) – and it fact, receptivity and selectivity may be mechanistically interrelated (as we will discuss below).
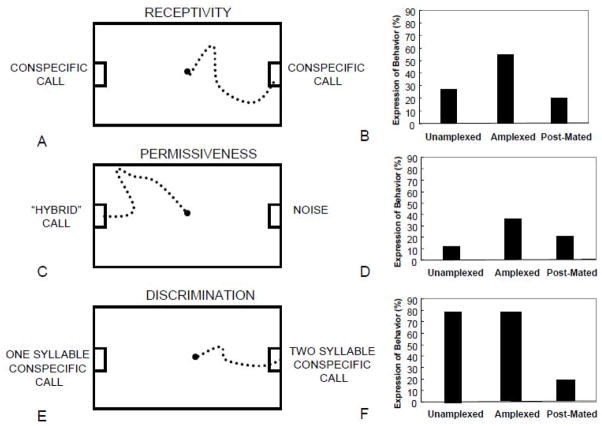
Lynch et al. (2005) used a series of phonotaxis tests of female túngara frogs (Physalaems pustulosus) to show that aspects of the female’s mate choice behavior change over a breeding cycle (Fig. 1). Females were field captured and tested in three natural states: when present at a breeding site but not in amplexus with a male; in amplexus with a male (indicating that she was ready to lay eggs and had begun behavioral interactions with a male of her choice), and after completing her egg laying. As measured by their willingness to approach any standard conspecific call, female receptivity was relatively low in the females prior to choosing a male, highest in females who were in amplexus when collected, then dropped to pre-amplexed levels after the female had dropped her eggs. The selectivity of females in these three states was assessed by presenting them just with a synthetic call that combined features of the conspecific P. pustulosus call with that of a heterospecific Physalaemus enesfee call to which females rarely respond. As receptivity increased, females became less choosy in this phonotaxis test: the proportion of female responses to this normally very unattractive call increased significantly, again dropping to pre-amplexus levels after the female dropped her eggs. While choosiness or selectivity fluctuated within a female, her ability to discern subtle differences among signals did not (Lynch et al. 2005; Lynch et al. 2006). When provided with two acceptable conspecific calls, the male’s two-syllable —whine-chuck and the male’s one syllable —whine, females at all stages of receptivity and choosiness picked the whine chuck regardless of how willing they were to show a phonotaxis response to a far more unattractive call when it was their only choice. This indicates that while subtle differences among signals remain noticeable to the female, they may not always be as meaningful to her (Nelson and Marler, 1990).
Figure 1.
Examples of phonotaxis tests in female túngara frogs at different reproductive stages indicating differences in sexual arousal. A: Diagram of receptivity test; conspecific call of some type is played from each speaker on either side of the test chamber. Female is considered receptive if she approaches either speaker. B: Percent of females expressing behavior when in the unamplexed state (prior to choosing a male), when in the amplexed stage (after choosing a male and initiating mating, when eggs are close to release), and after mating. C: Diagram of permissiveness test; a synthetic call melding conspecific and heterospecific acoustic elements usually yielding low responses is played from one speaker and noise from the other. Female permissiveness is measured by how likely she is to approach the synthetic —hybrid call. D: Percent of females expressing behavior when in the unamplexed state (prior to choosing a male), when in the amplexed stage (after choosing a male and initiating mating, when eggs are close to release), and after mating. Permissiveness increases when receptivity is high. E: Diagram of discrimination test; attractive 2-syllable conspecific call is played from one speaker and a less attractive 1-syllable call is played from the other to test female’s ability to discern differences in conspecific calls. F: Percent of females choosing the 2 syllable call is high in both the unamplexed and amplexed stages indicating that the receptivity and permissiveness results are not due to a difference in an ability to detect call differences. Discrimination decreases after mating when receptivity is low and female choices become inconsistent. Data are taken from Lynch et al., 2005. See that paper for further description of experimental design and controls procedures.
One function of this increase in receptivity and coincident decrease in choosiness may be to accommodate the female’s changing reproductive condition. Female anurans generally ovulate and develop eggs over several days during the breeding season (in túngara frogs, this takes 30–40 days ; Davidson and Hough, 1969) until they reach a point when their egg mass is large and ready to be released. At that point, they select a (calling) male, enter amplexus, and cooperate with the male in external fertilization of the released eggs. Túngara frog males also produce a foam nest for the eggs as they are released, and this nest is necessary for the eggs’ survival. If the female does not find a mate, it is possible that her eggs will have to be released anyway, and if so, her considerable reproductive effort will be lost. Female anurans that, like túngara frogs, can not reabsorb their eggs are thus faced with a serous time constraint that may cause them to trade -off their strong preferences for the most immediate opportunity to fertilize their eggs. Reproductive condition and time constraints related to it have been shown to influence mate choice behavior in other anurans (Lea et al. 2000; Bosch and Boyero 2004), as well as in a variety of other vertebrates such as fish (Forsgren, 1997; Kodric-Brownand Nicoletto, 2001 ) and birds (Qvarnström et al., 2000; Veen et al., 2001), as well as in invertebrates (Moore and Moore; 2001).
Hormones and reproductive behavior in female amphibians
There is converging evidence that anuran male sexual behaviors, such as production of advertisement mating signals, fluctuate in a seasonal manner coincident with fluctuations in reproductive hormone profiles (Licht et al., 1983; Mendonça et al., 1985; Rastogi et al, 1986 ; Itoh and Ishii, 1990). Furthermore, within a season the level of call production in male anurans is to some extent related to sex steroid levels (Wetzel and Kelley, 1983; Mendonça et al., 1985; Burmeister and Wilczynski, 2001; Emerson and Hess, 2001), peptide hormones (Boyd, 1994; Chu et al., 1998 ; Burmeister et al., 2001; Ten Eyck, 2005; Trainor et al., 2003 ; Kime et al., 2007)and gonadal state (Brzoska and Obert, 1980). Similarly, female receptivity is seasonal and related to gonadal steroids. Early studies of female anuran sexual behavior used pharmacological hormone manipulation to induce sexually receptive behaviors such as phonotaxis or mate calling in female Xenopus lavealis. These studies report that intraperitoneal administration of human chorionic gonadotropin (HCG) or other gonadotropins are effective at inducing receptive behaviors (Kelley, 1982; Schmidt, 1984). HCG is a gonadotropin that causes a general elevation in sex steroid hormones rather than specifically targeting one hormone. Studies such as these examined whether or not administration of hormones increase motivation to mate (i.e. receptivity) by measuring if the hormone administration resulted in either an increase in phonotatic responses to conspecific mate calls (Lynch et al., 2006; Chakraborty and Burmeister, 2009; Gordon and Gerhardt, 2009)a decrease in the latency to respond (Boyd, 1994) or an inhibition of unreceptive behaviors such as release calls or leg extensions (Kelley, 1982). They did not address the question of selectivity in mate responses. Other hormones also contribute to modulating female receptivity. Sex steroids may exert greater effects on receptive responses in female anurans if administered in combination with peptide hormones such as luteinizing hormone-releasing hormone (Kelley, 1982) or lipid-based hormones such as prostaglandins (Schmidt, 1985). Some studies report that administration of peptide hormones such as arginine vasotocin or prostaglandins will alone induce phonotaxis responses and inhibit release calls respectively (Diakow and Nemiroff, 1981; Weintraub et al., 1985).
As is the case for many vertebrates, receptive behavior in female anurans is typically expressed when both estrogen and progesterone are naturally elevated in wild-caught anurans (Pierantoni et al., 1984; Iela et al., 1986; Harvey et al., 1997; Medina et al., 2004; Lynch and Wilczynski, 2005). Female anurans exhibit elevated progesterone and estrogen as they near the time at which they oviposit (Harvey et al., 1997; Medina et al., 2004; Lynch and Wilczynski, 2005). This is also the time at which the females express positive phonotatic responses. A recent study reported that female gray treefrogs (Hyla versicolor) exhibited a significant increase in phonotatic responses after treatment with progesterone plus prostaglandin (Gordon and Gerhardt, 2009 ). The authors report, however, that the progesterone-prostaglandin treatment significantly elevated estrogen levels over non -treated animals. Thus it is possible that the progesterone -prostaglandin injection induces elevated estrogen concentrations in the plasma, and it is this estrogen that is necessary and possibly sufficient for the induction of phonotatic responses. In fact, Chakroborty and Burmeister (2009) administered HCG, estradiol, or estradiol plus progesterone to different groups of female tungara frogs. The authors report that all of these groups of hormone manipulated females exhibited elevated phonotatic response in comparison to saline treatments. Furthermore, HCG injected females treated with an aromatase inhibitor failed to show an increase in phonotatic responses. This suggests that estrogen in indeed necessary and sufficient to evoke phonotatic responses in female anurans. Future tests should reveal if this is a consistent pattern across various amphibian species, but the work to date does suggest that estrogen is an important hormonal regulator of female receptivity.
As in anurans, female reproductive behavior in urodeles is regulated by peptide and steroid hormones. For instance in terrestrial-phase females the migration to the water where reproduction occurs is prolactin-dependent (Iwata et al., 2000; Chadwick, 1941). The hormonal secretions that females release to allow conspecific males to identify her as sexually responsive (Kikuyama et al., 2009 for review) can be induced via prolactin and estradiol treatments (Toyoda et al., 1994). Not only are the female’s pheromone secretions hormonally regulated but her response to the male’s courtship pheromone is also hormonally regulated. Female red-bellied newts must be treated with prolactin and gonadotropin in order to exhibit behavioral responses to water in which male newts have released a female-attracting pheromone (Toyoda, 1994). Furthermore, the elevated prolactin and estradiol not only serve to elicit attractant-like secretions from the female, these hormones also elicit oviductal development (Kikuyama et al., 1986). Consequently, these hormones coordinate the female’s behavioral response to courting males with physiological changes that must occur in order for her to successfully signal her own receptivity and to successfully reproduce.
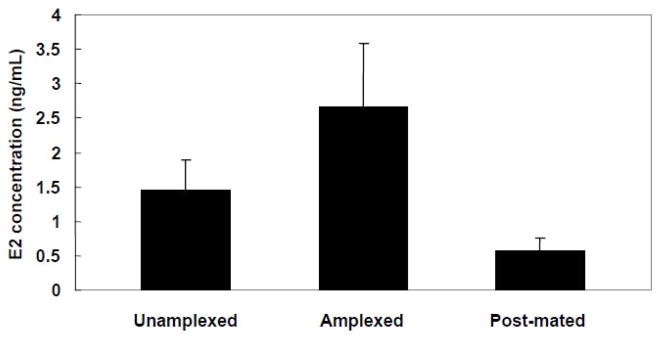
Lynch and Wilczynski (2005) found that estrogen levels cycle during the túngara frog female’s reproductive cycle in a manner reminiscent of the estrous cycling of mammals (Fig 2). The estrogen variation maps onto the behavioral variation seen in these females. Estrogen is highest in females captured in amplexus when presumably they are closest to laying their eggs. At this point, receptivity is highest and choosiness lowest. Estrogen, along with both behaviors, falls after eggs are released. Manipulating gonadal steroids through HCG treatment manipulated receptivity and choosiness in the predicted direction (Lynch and Wilczynski, 2006). It therefore seems as though the episodic changes in receptivity and choosiness that serve as indicators of changes in female sexual arousal are tied mechanistically to gonadal steroid changes in amphibians. If sexual arousal in amphibians is analogous to mammalian sexual arousal, a sensory manipulation of the processes driving these changes would occur. In fact, there is evidence that male signals can elevate the gonadal steroids in receivers.
Figure 2.
Level of plasma estrogen in female túngara frogs prior to interacting with a male (unamplexed), in the amplexed stage when eggs are close to release and females have chosen a male for mating, and after mating and egg release. Compare the hormone pattern to the behavioral patterns shown in Figure 1. From Lynch and Wilczynski, 2005.
Male signals elevate reproductive physiology
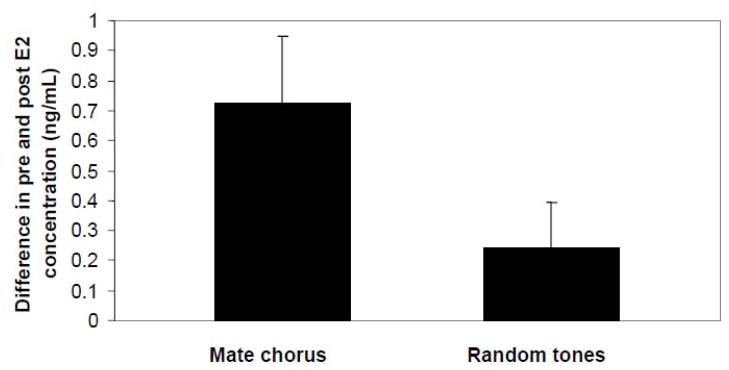
Daniel Lehrman’s classic work on ring doves (Lehrman, 1965) focused behavioral endocrinologists on the reciprocal influences that hormones and behavior have on each other. Although this work has never been articulated as an example of female sexual arousal, in fact the courtship vocalizations between males and females, and the linked behavioral and hormonal changes that follow and lead to the female accepting the copulations of males do fulfill the criteria for thinking about animal social interactions in this way. Since Lehrman’s work, many other studies have shown that stimulatory signals by males induce receptivity in females on a variety of time scales, from bringing females into estrous more quickly (e.g., McComb’s 1987 demonstration that the roars of red deer males advance estrous in females) to the more immediate role that the male tactile and chemical signals have in inducing lodorsis responses in female rats (Pfaff et al., 1973; Noble, 1973; Beach et al., 1976). In amphibians, the first demonstrations that social signals can stimulate reproductive hormone secretion were in males. Brzoska and Obert (1984) found that male Rana esculenta that heard conspecific calls maintained larger testes than did control males. Direct hormone measures of circulating testosterone found that males of both Rana sphenocephala (Chu and Wilczynski, 2001) and Hyla cinerea (Burmeister and Wilczynski, 2000, 2001) increased androgen levels after hearing conspecific calls for several days. Anatomical and electrophysiological studies in fact show an "audioendocrine" circuit (Wilczynski and Endepols, 2007) from the midbrain auditory center to basal forebrain areas regulating the endocrine system. Stimulatory effects of male vocal signals on female reproductive state have now been demonstrated in amphibians. Lea et al. (2001) found that male calls changed female reproductive condition in midwife toads (Alytes muletensis). Some female midwife toads were exposed to conspecific male calls whereas other groups of females heard heterospecifc male calls or nothing at all. The females exposed to conspecific calls continued to mature their eggs whereas the eggs of females in the other groups were reabsorbed. This was the first study to report that the male courtship calls could have a stimulatory effect on the reproductive axis of the listening female. In a later study, Lynch and Wilczynski (2006) measured estrogen levels in female túngara frogs and found that hearing conspecific calls for 10 consecutive nights significantly increased plasma estrogen levels compared to control females (Fig. 3). These results are significant in the context of female sexual arousal. Male signals elevate female estrogen; elevated estrogen is correlated with increased female receptivity and decreased female choosiness, both increasing the female’s willingness to accept the sexual overtures of the male.
Figure 3.
Change in plasma estrogen in female túngara frogs hearing conspecific male vocal signals vs. random tones (matched for duration and amplitude) for ten consecutive nights. Hearing male signals significantly increases estrogen. From Lynch and Wilczynski, 2006.
It is important to note that the work on male stimulation of female reproductive state in anurans has so far focused on a time line that is much longer than the immediate behavioral stimulation of female approaches that the male vocal signals trigger. As yet there is little known about other, more immediate sensory cues that male anurans might produce during the amplexus stage, or period immediately leading up to it, that could stimulate females to their final state of arousal leading to egg release. Clues might be taken from work on urodeles, where males and females interact tactile and chemically during a close courtship interaction that eventually leads to the female accepting the male’s spermatophore (see Kikuyama et al. 2009 for a general review of urodele mating behavior). These interactions are more similar to the kind of male-female interaction in rodents leading to lordosis and copulation. On the other hand, our understanding of hormonal or other physiological change in the urodele female as a result of the male courtship is limited. To date it has only been reported that GnRH concentrations within the anterior telencephalon were elevated in female newts that were unreceptive and exposed to courting males. The GnRH concentrations declined again after the female exhibited receptive behavior by accepting the male’s spermatophore. Furthermore, courted, receptive females had higher plasma levels of estradiol than did uncourted controls (Propper and Moore, 1991). These alterations in peptide and steroid hormones are thought to be a consequence of social interactions with courting males. However, there is still little known about these physiological changes.
Perceptual modification as a mechanism of female sexual arousal in amphibians
One possible means by which hormones might enhance female behavioral responses to male mate signals is by modifying the manner in which the female processes the male’s advertisement call. That is, the female’s move toward greater receptivity and diminished choosiness to the male vocal signals (while maintaining the ability to discriminate among calls) could be the consequence of a change in how those signals are processed in the central nervous system. Although we cannot really know how a female frog perceives a signal, or how a physiological state change causes her to —feel about male sexual signals, a variety of evidence can be assembled to suggest that changes in estrogen yield changes in the ability of male vocal signals to stimulate a key auditory midbrain area, the torus semicircularis, apart of the ascending auditory system that is homologous with the mammalian inferior colliculus (Wilczynski and Endepols, 2007).
The torus is a key center responsible for relaying ascending auditory information to multiple areas of the forebrain responsible that contribute to both the behavioral and the endocrine responses to social (and other) cues through two of its subnuclei, the principal and laminar nucleus (PN or LN respectively) (Wilczynski et al., 1993; Wilczynski and Endepols, 2007) The torus contains steroid-binding sites in Xenopus laevis, Rana esculenta, Physaleamus pustulosus (di Megli et al. 1987; Kelley et al., 1975, 1978; Morrell et al., 1975; Kelley, 1980; Endepols et al., 2000; Chakraborty and Burmeister, 2010). These steroid-binding sites present the possibility for hormonally -modulated auditory response properties. In fact, earlier studies have shown seasonal fluctuations in the electrophysiological responses of TS neurons, which were assumed to provide an indication of hormonal sensitivity (Walkowiak, 1980; Hillery, 1984; Goense and Feng 2005), and one electrophysiologial study found that estrogen treatment did lower toral thresholds to acoustic stimulation (Yovanof and Feng, 1983 ). A more recent study on Hyla cinerea females found that the neurophysiological response properties of toral neurons not only exhibit plasticity between seasons but within seasons as well in a manner related to female reproductive condition (Miranda and Wilczynski, 2009 ). In this report, neural response strength to tones was significantly elevated in females prior to mating as compared to females that had already mated. In a time line similar to the drop in estrogen reported by Lynch and Wilczynski (2005) in a different species, postmating toral responses rapidly declined. These results show that the effect of acoustic signals on auditory neurons, at least at the level of the midbrain, is dependent upon the reproductive state of the female, and that neural stimulation by acoustic signals is stronger when estrogen levels are high and during the same period of the reproductive cycle when receptivity is high.
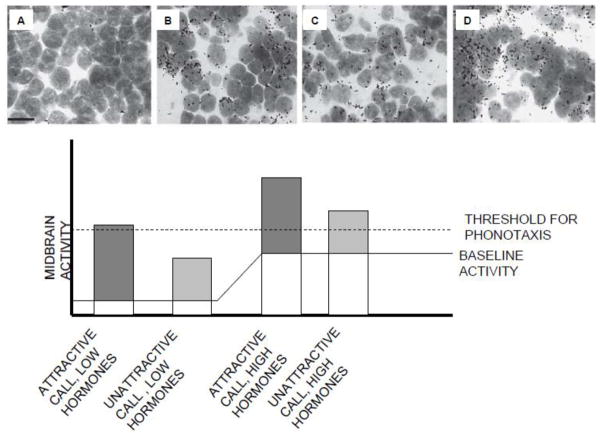
In a recent study, Lynch and Wilczynski (2008) used expression of the immediate early gene egr-1 to more directly assay the effect of hormone state on toral activity to conspecific calls intúngara frogs (Fig. 4). In a 2×2 design females were exposed to mate chorus sounds or silence, and treated with HCG or a saline vehicle. The HCG significantly elevated estrogen levels in treated female. The silence+vehicle condition has the lowest level of ieg expression. The laminar nucleus (LN)of the torus exhibited a similar level of elevated egr-1 induction in response to mate chorus exposure and gonadotropin treatment. The gonadotropin + chorus exposure had the highest expression. This suggests that mate signals and hormones together produce an additive effect so that together they induce more egr-1 expression, presumably indicating higher neural activity, than either alone. This hormonally modulated sensory processing may serve as a means to increase the female’s stimulation by male advertisement calls and thus her receptivity towards them. Interestingly, the general increase induced by the elevated gonadal hormones should also allow otherwise unattractive (i.e., low stimulation) signals to reach the threshold for triggering a phonotaxis response, but would also provide sufficient differential activation to allow females to discriminate the relative attractiveness of competing calls. The egr-1 results provide a measure of midbrain physiological activation that is consistent with the behavioral variation one sees at different endocrinological states in females of this species.
Figure 4.
Top A–D: Expression of the immediate early gene egr-1 in the midbrain (laminar nucleus of the torus semicircularis) in a female túngara frog under conditions of low estrogen + no call stimulation, low estrogen + conspecific call stimulation, high estrogen + no call stimulation, and high estrogen + conspecific cal stimulation respectively. Bottom: Suggested model of how elevated estrogen and stimulation by various calls interact in stimulating the midbrain. White bars indicate baseline midbrain activity due to hormone action, gray bars indicate midbrain activity due to call stimulation. When hormone levels are low, attractive calls may reach threshold for triggering a phonotaxis response, but unattractive calls fail to reach threshold. When hormone levels are high, both call types could reach threshold for triggering a phonotaxis response. Behavioral, this would appear to be an increase in both receptivity and permissiveness in the female, with preservation of her ability to discriminate calls based on the level of activation. Based on figures in Lynch and Wilczynski, 2008.
Taken together, the studies reviewed here suggest a novel mechanism by which male anurans may increase their chances of evoking a receptive behavioral response from a female, that is, increasing her sexual arousal. First, social cues (male advertisement calls) increase estrogen levels in the female hearing them. Estrogen modifies the perception of the signal itself by increasing it s net stimulation of neural responses in a key auditory center responsible for relaying information to the forebrain. This increase in neural stimulation increases the probability of phonotactic responses to male calls (that is, increases receptivity) while broadening the range of possible signals that would stimulate such a response (decreases choosiness). Whether estrogen also increases motivation to respond (receptivity) to male calls independently by acting on other brain areas, or only as a consequence of altered sensory processing remains, to be seen.
Changes in sensory processing may also underlie changes in female urodele arousal. Treatment with either prolactin or estrogen increases the responsiveness of cells in the vomeronasal organ epithelium to male pheromone (Toyoda and Kikuyama, 2000). It is therefore possible that this hormone-regulated sensory modulation may facilitate the behavioral responses of female newts to male chemical courtship signals in the same way that elevated estrogen facilitates the responses of the female midbrain to male vocal signals.
Sexual arousal in female amphibians
None of the various studies reviewed above on variation in female amphibian reproductive behavior specifically raised the topic of —sexual arousal. But in fact, the situationally-dependent changes in female responses can be seen as examples of that phenomenon. Females have both behavioral and endocrine changes that make their reception of male courtship advances more likely. These changes may result from internal signals related to egg maturation, but it is clear that stimulation by male sexual signals also play a role. The responses make mating with a male more likely, and are then terminated (or more accurately, the elevated receptivity returns to baseline) quickly after sexual interactions between males and females are consummated. The hormonal mechanisms underlying the female variation are similar to those seen across vertebrates, with estrogens playing an important role and a variety of other hormones contributing to the switch from an unreceptive to a receptive state.
It is the case that the timing of the changes in anuran amphibians, especially the hormone changes, are different than what one normally associates with situational female sexual arousal, being much slower and not, as far is as known at present, rapid and closely tied to the immediate presence of a male. The behavioral courtship interactions in urodeles may fit the traditional pattern more closely. However, it is the case that we know very little about the more rapid signaling between male and female anurans immediately before they enter amplexus or about such signaling during the amplexus period, and any consequent behavioral or physiological changes that may occur during either of these parts of their reproductive interaction. Understanding those would provide a fuller picture of the control of female sexual arousal in this taxonomic group.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beach FA. Sexual attractivity, proceptivity, and receptivity in female mammals. Horm Behav. 1976;7:105–138. doi: 10.1016/0018-506x(76)90008-8. [DOI] [PubMed] [Google Scholar]
- Bee MA. Finding a mate at a cocktail party: Spatial release from masking improves acoustic mate recognition in grey treefrogs. Anim Behav. 2008;75:1781–1791. doi: 10.1016/j.anbehav.2007.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch J, Boyero L. Reproductive stage and phonotatic preferences of female midwife toads (Alytes cisternasii) Behav Ecol Sociobiol. 2004;55:251–256. [Google Scholar]
- Boyd SK. Sexual differences in hormonal control of release calls in bullfrogs. Horm Behav. 1992;26:522–535. doi: 10.1016/0018-506x(92)90019-r. [DOI] [PubMed] [Google Scholar]
- Boyd SK. Arginine vasotocin facilitation of advertisement calling and call phonotaxis in bullfrogs. Horm Behav. 1994;28:232–240. doi: 10.1006/hbeh.1994.1020. [DOI] [PubMed] [Google Scholar]
- Brzoska J, Obert HJ. Acoustic signals influence the hormone production of the testes in the grass frog. J Comp Physiol. 1980;140:25–29. [Google Scholar]
- Burmeister SS, Wilczynski W. Social signals influence hormones independently of calling behavior in the treefrog (Hyla cinerea) Horm Behav. 2000;38:201–209. doi: 10.1006/hbeh.2000.1605. [DOI] [PubMed] [Google Scholar]
- Burmeister SS, Wilczynski W. Social context influences androgenic effects on calling in the green treefrog (Hyla cinerea) Horm Behav. 2001;40:550–558. doi: 10.1006/hbeh.2001.1723. [DOI] [PubMed] [Google Scholar]
- Chadwick CS. Further observation on the water drive in Triturus viridescensII. Induction of the water drive with the lactogenic hormone. J Exp Zool. 1941;86:175–187. [Google Scholar]
- Chakraborty M, Burmeister SS. Estradiol induces sexual behavior in female túngara frogs. Horm Behav. 2009;55:106–112. doi: 10.1016/j.yhbeh.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Chakraborty M, Burmeister SS. Sexually dimorphic androgen and estrogen receptor mRNA expression in the brain of túngara frogs. Horm Behav. 2010 doi: 10.1016/j.yhbeh.2010.06.013. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Chu J, Marler CA, Wilczynski W. The effects of arginine vasotocin on the calling behavior of male cricket frogs in changing social context. Horm Behav. 1998;34:248–261. doi: 10.1006/hbeh.1998.1479. [DOI] [PubMed] [Google Scholar]
- Chu J, Wilczynski W. Social influences on androgen levels in the southern leopard frog, Rana sphenocephala. Gen Comp Endocrinol. 2001;121:66–73. doi: 10.1006/gcen.2000.7563. [DOI] [PubMed] [Google Scholar]
- Davidson EH, Hough BR. Synchronous oogenesis in Engystomops pustulosus, a neotropic anuran suitable for laboratory studies: localization in the embryo of RNA synthesized at the lampbrush stage. J Exp Zool. 1969;172:25–48. doi: 10.1002/jez.1401720104. [DOI] [PubMed] [Google Scholar]
- Diakow C. Hormonal basis for breeding behavior in female frogs: vasotocin inhibits the release call of Rana pipiens. Science. 1978;199:1456–1457. doi: 10.1126/science.305115. [DOI] [PubMed] [Google Scholar]
- Diakow C, Nemiroff A. Vasotocin, prostaglandin and female reproductive behavior in the frog, Rana pipiens. Horm Behav. 1981;15:86–93. doi: 10.1016/0018-506x(81)90037-4. [DOI] [PubMed] [Google Scholar]
- di Megli M, Morrell JI, Pfaff DW. Localization of steroid-concentrating cells in the central nervous system of the frog Rana esculenta. Gen Comp Endocrinol. 1987;67:149–154. doi: 10.1016/0016-6480(87)90142-0. [DOI] [PubMed] [Google Scholar]
- Elliott TM, Kelley D. Male discrimination of receptive and unreceptive female calls by temporal features. J Exp Bio. 2007;210:2836–2842. doi: 10.1242/jeb.003988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson SB, Hess DL. The role of androgens in opportunistic breeding, tropical frogs. Gen Comp Endocrinol. 1996;103:220–230. doi: 10.1006/gcen.1996.0113. [DOI] [PubMed] [Google Scholar]
- Endepols H, Walkowiak W, Luksch H. Chemoarchitecture of the anuran auditory midbrain. Brain Res Rev. 2000;33:179–198. doi: 10.1016/s0165-0173(00)00029-1. [DOI] [PubMed] [Google Scholar]
- Forsgren E. Mate sampling in a population of sand gobies. Anim Behav. 1997;53:267–276. [Google Scholar]
- Gerhardt HC, Huber F. Acoustic Communication in Insects and Anurans. University of Chicago Press; Chicago: 2002. [Google Scholar]
- Goense JB, Feng AS. Seasonal changes in frequency tuning and temporal processing in single neurons in the frog auditory midbrain. J Neurobiol. 2005;65:22–36. doi: 10.1002/neu.20172. [DOI] [PubMed] [Google Scholar]
- Gordon NM, Gerhardt HC. Hormonal modulation of phonotaxis and advertisement-call preferences in the gray treefrog (Hyla versicolor) Horm Behav. 2009;55:121–127. doi: 10.1016/j.yhbeh.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey LA, Propper CR, Woodley SK, Moore MC. Reproductive endocrinology of the explosively breeding desert spadefoot toad, Scaphiopus couchii. Gen Comp Endocrinol. 1997;105:102–113. doi: 10.1006/gcen.1996.6805. [DOI] [PubMed] [Google Scholar]
- Hillery CM. Seasonality of two midbrain auditory responses in the treefrog Hyla chrysoscelis. Copeia. 1984;1984:844–852. [Google Scholar]
- Iela L, Rastogi RK, Delrio G, Bagnara JT. Reproduction in the Mexican leaf frog, Pachymedusa dacnicolor III. The female. Gen Comp Endocrinol. 1986;63:381–392. doi: 10.1016/0016-6480(86)90137-1. [DOI] [PubMed] [Google Scholar]
- Itoh M, Ishii S. Changes in plasma levels of gonadotropins and sex steroids in the toad, Bufo japonicus, in association with behavior during breeding season. Gen Comp Endocrinol. 1990;80:451–464. doi: 10.1016/0016-6480(90)90194-q. [DOI] [PubMed] [Google Scholar]
- Iwata T, Toyoda F, Yamamoto K, Kikuyama S. Hormonal control of urodele reproductive behavior. Comp Biochem Phys. 2000;B 126:221–229. doi: 10.1016/s0305-0491(00)00200-5. [DOI] [PubMed] [Google Scholar]
- Jennions MD, Petrie M. Variation in mate choice and mating preferences: a review of causes and consequences. Biol Rev. 1997;72:283–327. doi: 10.1017/s0006323196005014. [DOI] [PubMed] [Google Scholar]
- Kelley DB, Morrell JI, Pfaff DW. Autoradiographic localization of hormone concentrating cells in the brain of an amphibian, Xenopus laevis. I. Testosterone. J Comp Neurol. 1975;164:47–59. doi: 10.1002/cne.901640105. [DOI] [PubMed] [Google Scholar]
- Kelley DB, Lieberburg I, McEwan BS, Pfaff DW. Autoradiographic and biochemical studies of steroid hormone-concentrating cells in the brain of Rana pipiens. Brain Res. 1978;140:287–305. doi: 10.1016/0006-8993(78)90461-4. [DOI] [PubMed] [Google Scholar]
- Kelley DB. Auditory and vocal nuclei in the frog brain concentrate sex hormones. Science. 1980;207:553–555. doi: 10.1126/science.7352269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley DB. Female sex behaviors in the south African clawed frog, Xenopus laevis: Gonadotropin-releasing, gonadotropic and steroid hormones. Horm Behav. 1982;16:158–174. doi: 10.1016/0018-506x(82)90016-2. [DOI] [PubMed] [Google Scholar]
- Kikyuyama S, Seshimo H, Shirama K, Kato T, Noumura T. Interaction of prolactin with sex steroid in oviduct and tail of newts, Cynops pyrrhogaster. Zool Sci. 1986;3:131–138. [Google Scholar]
- Kikuyama S, Hasunuma I, Toyoda F, Haraguchi S, Tsutsui K. Hormone -mediated reproductive behavior in the Red-bellied newt. Trends in Comparative Endocrinology and Neurobiology. Ann NY Acad Sci. 2009;1163:179–186. doi: 10.1111/j.1749-6632.2009.04449.x. [DOI] [PubMed] [Google Scholar]
- Kime NM, Whitney TK, Davis ES, Marler CA. Arginine vasotocin promotes calling behavior and call changes in male túngara frogs. Brain Behav Evol. 2007;69:254–65. doi: 10.1159/000099613. [DOI] [PubMed] [Google Scholar]
- Kodric-Brown A, Nicoletto PF. Age and experience affect female choice in the guppy (Poecilia reticulate) Am Nat. 2001;157:316–323. doi: 10.1086/319191. [DOI] [PubMed] [Google Scholar]
- Lea J, Dyson M, Halliday T. Calling by male midwife toads stimulates females to maintain reproductive condition. Anim Behav. 2001;61:373–377. [Google Scholar]
- Lea J, Halliday T, Dyson M. Reproductive stage and history affect the phonotactic preferences of female midwife toads, Alytes muletensis. Anim Behav. 2000;60:423–427. doi: 10.1006/anbe.2000.1482. [DOI] [PubMed] [Google Scholar]
- Lehrman DS. Interaction between internal and external environments in the regulation of the reproductive cycle of the ring dove. In: Beach FA, editor. Sex and Behavior. New York: Wiley; 1965. pp. 344–380. [Google Scholar]
- Licht P, McCreery BR, Barnes R, Pang R. Seasonal and stress related changes in plasma gonadotropins, sex steroids and corticosterone in the bullfrog, Rana catesbeiana. Gen Comp Endocrinol. 1983;50:124–145. doi: 10.1016/0016-6480(83)90249-6. [DOI] [PubMed] [Google Scholar]
- Lynch KS, Crews DC, Ryan MJ, Wilczynski W. Hormonal state influences aspects of female mate choice in the túngara frog (Physalaemus pustulosus) Horm Behav. 2006;49:450–457. doi: 10.1016/j.yhbeh.2005.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch KS, Rand AS, Ryan MJ, Wilczynski W. Plasticity in female mate choice associated with changing reproductive states. Anim Behav. 2005;69:689–699. [Google Scholar]
- Lynch KS, Wilczynski W. Gonadal steroids vary with reproductive stage in a tropically breeding female Anuran. Gen Comp Endocrinol. 2005;143:51–66. doi: 10.1016/j.ygcen.2005.02.023. [DOI] [PubMed] [Google Scholar]
- Lynch KS, Wilczynski W. Social regulation of plasma estradiol concentration in a female anuran. Horm Behav. 2006;50:101–106. doi: 10.1016/j.yhbeh.2006.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch KS, Wilczynski W. Reproductive hormones modify reception of species-typical communication signals in a female anuran. Brain Behav Evol. 2008;71:143–50. doi: 10.1159/000111460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McComb K. Roaring by red deer stags advances the date of oestrus in hinds. Nature. 1987;330:648–649. doi: 10.1038/330648a0. [DOI] [PubMed] [Google Scholar]
- Medina MF, Ramos I, Crespo CA, González-Calvar S, Fernández SN. Changes in serum sex steroid levels throughout the reproductive cycle of Bufo arenarum females. Gen Comp Endocrinol. 2004;136:143–151. doi: 10.1016/j.ygcen.2003.11.013. [DOI] [PubMed] [Google Scholar]
- Mendonça MT, Licht P, Ryan MJ, Barnes R. Changes in hormone levels in relation to breeding behavior in male bullfrogs ( Rana catesbeiana) at the individual and population levels. Gen Comp Endocrinol. 1985;58:270–279. doi: 10.1016/0016-6480(85)90343-0. [DOI] [PubMed] [Google Scholar]
- Miranda JA, Wilczynski W. Female reproductive state influences the auditory midbrain response. J Comp Physiol A. 2009;195:341–349. doi: 10.1007/s00359-008-0410-7. [DOI] [PubMed] [Google Scholar]
- Moore PJ, Moore AJ. Reproductive aging and mating: the ticking of the biological clock in female cockroaches. Proc Natl Acad Sci U S A. 2001;98:9171–9176. doi: 10.1073/pnas.161154598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore FL, Wood RE, Boyd SK. Sex steroids and vasotocin interact in a female amphibian (Taricha granulosa) to elicit female-like egg-laying behavior or male-like courtship. Horm Behav. 1992;26:156–166. doi: 10.1016/0018-506x(92)90039-x. [DOI] [PubMed] [Google Scholar]
- Morrell JI, Kelley DB, Pfaff DW. Autoradiographic localization of hormone-concentrating cells in the brain of an amphibian, Xenopus laevis. II. Estradiol. J Comp Neurol. 1975;164:63–77. doi: 10.1002/cne.901640106. [DOI] [PubMed] [Google Scholar]
- Nelson DA, Marler P. The perception of bird song and an ecological concept of signal space. In: Stebbins WC, Berkeley MA, editors. Comparative Perception. New York: J. Wiley; 1990. pp. 443–478. [Google Scholar]
- Noble RG. Sexual arousal of the female hamster. Brain Res. 1973;10:973–975. doi: 10.1016/0031-9384(73)90070-x. [DOI] [PubMed] [Google Scholar]
- Pfaff D, Lewis C, Diakow C. Neurophysiological analysis of mating behavior responses as hormone-sensitive reflexes. Prog Physiol Psych. 1973;5:253–297. [Google Scholar]
- Pierantoni R, Iela L, Delrio G, Rastogi RK. Seasonal plasma sex steroid levels in the female Rana esculenta. Gen Comp Endocrinol. 1984;53:126–134. doi: 10.1016/0016-6480(84)90232-6. [DOI] [PubMed] [Google Scholar]
- Propper CR, Moore FL. Effects of courtship on brain gonadotropin hormone releasing hormone and plasma steroid concentrations in a female amphibian (Taricha granulosa) Gen Comp Endocrinol. 1991;81:304–312. doi: 10.1016/0016-6480(91)90015-x. [DOI] [PubMed] [Google Scholar]
- Qvarnstrom A, Pärt T, Sheldon BC. Adaptive plasticity in mate preferences linked to differences in reproductive effort. Nature. 2000;405:344–347. doi: 10.1038/35012605. [DOI] [PubMed] [Google Scholar]
- Rastogi RK, Iela L, Delrio G, Bagnara JT. Reproduction in the Mexican leaf frog, Pachymedusa dacnicolor. II. The male. Gen Comp Endocrinol. 1986;62:23–35. doi: 10.1016/0016-6480(86)90090-0. [DOI] [PubMed] [Google Scholar]
- Ryan MJ, Rand W, Hurd PL, Phelps SM, Rand AS. Generalization in response to mate recognition signals. Am Nat. 2003;161:380–94. doi: 10.1086/367588. [DOI] [PubMed] [Google Scholar]
- Ryan MJ. Female mate choice in a neotropical frog. Science. 1980;209:523–525. doi: 10.1126/science.209.4455.523. [DOI] [PubMed] [Google Scholar]
- Ryan MJ. The Túngara Frog: a Study in Sexual Selection and Communication. University of Chicago Press; Chicago: 1985. [Google Scholar]
- Ryan MJ. Sexual selection and mate choice. In: Krebs JR, Davies NB, editors. Behavioral ecology: an Evolutionary Approach. Blackwell; Oxford: 1997. pp. 179–202. [Google Scholar]
- Schmidt RS. Mating call phonotaxis in the female American toad: induction by hormones. Gen Comp Endocrinol. 1984;55:150–156. doi: 10.1016/0016-6480(84)90139-4. [DOI] [PubMed] [Google Scholar]
- Schmidt RS. Prostaglandin-induced mating call phonotaxis in female American toad: facilitation by progesterone and arginine vasotocin. J Comp Physiol A. 1985;156:823–829. [Google Scholar]
- Ten Eyck GR. Arginine vasotocin activates advertisement calling and movement in the territorial Puerto Rican frog, Eleutherodactylus coqui. Horm Behav. 2005;47:223–229. doi: 10.1016/j.yhbeh.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Tobias ML, Viswanathan SS, Kelley DB. Rapping, a female receptive call, initiates male-female duets in the South African clawed frog. Proc Nat Acad Sci USA. 1998;95:1970–1975. doi: 10.1073/pnas.95.4.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda F, Kikuyama S. Hormonal influence on the olfactory response to a female-attracting pheromone, sodefrin, in the newt, Cynops pyrrhogaster. Comp Biochem Physiol B Biochem Mol Biol. 2000;126:239–245. doi: 10.1016/s0305-0491(00)00202-9. [DOI] [PubMed] [Google Scholar]
- Toyoda F, Tanaka S, Matsuda K, Kikuyama S. Hormonal control of response to and secretion of sex attractants in Japanese newts. Physiol Behav. 1994;55:569–576. doi: 10.1016/0031-9384(94)90118-x. [DOI] [PubMed] [Google Scholar]
- Trainor BC, Rouse KL, Marler CA. Arginine vasotocin interacts with the social environment to regulate advertisement calling in the gray treefrog (Hyla versicolor) Brain Behav Evol. 2003;61:165–171. doi: 10.1159/000070700. [DOI] [PubMed] [Google Scholar]
- Veen T, Borge T, Griffith SC, Sætre GP, Bures S, Gustafsson L, Sheldon BC. Artificial hybridization and adaptive mate choice in flycatchers. Nature. 2001;411:45–50. doi: 10.1038/35075000. [DOI] [PubMed] [Google Scholar]
- Walkowiak W. The coding of auditory signals in the torus semicircularis of the fire-bellied toad and the grass frog—responses to simple stimuli and to conspecific calls. J Comp Physiol A. 1980;138:131–148. [Google Scholar]
- Weintraub AS, Kelley DB, Bockman RS. Prostaglandin E2 induces receptive behaviors in female Xenopus laevis. Horm Behav. 1985;19:386–399. doi: 10.1016/0018-506x(85)90036-4. [DOI] [PubMed] [Google Scholar]
- Wells KD. The social behaviour of anuran amphibians. Anim Behav. 1977;25:666–693. [Google Scholar]
- Wetzel DM, Kelley DB. Androgen and gonadotropin effects on male mate calls in South African clawed frogs, Xenopus laevis. Horm Behav. 1983;17:388–404. doi: 10.1016/0018-506x(83)90048-x. [DOI] [PubMed] [Google Scholar]
- Wilczynski W, Allison JD, Marler CA. Sensory pathways linking social and environmental cues to endocrine control regions of amphibian forebrains. Brain Behav Evol. 1993;42:252–264. doi: 10.1159/000114159. [DOI] [PubMed] [Google Scholar]
- Wilczynski W, Endepols H. Central auditory pathways in anuran amphibians: The anatomical basis of hearing and sound communication. In: Popper AN, Feng AS, Narins PN, editors. Hearing and Sound Communication in Amphibians: Springer Handbook of Auditory Research. Vol. 28. Berlin: Springer-Verlag; 2007. [Google Scholar]
- Yovanof S, Feng AS. Effects of estradiol on auditory evoked responses from the frog’s midbrain. Neurosci Letters. 1983;36:291–297. doi: 10.1016/0304-3940(83)90015-0. [DOI] [PubMed] [Google Scholar]