Table 1.
Screening of the catalysts and optimization of the reaction conditions[a]
| Entry | Catalyst | Acid | Solvent | Time [d] | Yield [%][b] | ee [%][c] |
|---|---|---|---|---|---|---|
| 1 | 1 | HCl | THF | 5 | 64 | 66 |
| 2 | 2 | HCl | THF | 5 | 63 | 50 |
| 3 | 3 | HCl | THF | 5 | 51 | 57[d] |
| 4 | 4 | HCl | THF | 8 | 21 | 40[d] |
| 5 | 5 | HCl | THF | 9 | trace | nd[e] |
| 6 | 6 | HCl | THF | 5 | 21 | 5 |
| 7 | 7 | HCl | THF | 6 | 56 | 3 |
| 8 | 1 | HCl | dioxane | 7 | 80 | 61 |
| 9 | 1 | HCl | CHCl3 | 5 | 60 | 59 |
| 10 | 1 | HCl | CH2Cl2 | 5 | 62 | 50 |
| 11 | 1 | HCl | toluene | 9 | 12 | 37 |
| 12 | 1 | HCl | TFE[f] | 5 | trace | nd[e] |
| 13 | 1 | HCl | CH3CN | 5 | 85 | 40 |
| 14 | 1 | HCl | DMSO | 5 | 83 | 17 |
| 15 | 1 | HCl | acetone | 5 | 43 | 46 |
| 16 | 1 | PhCO2H | THF | 15 | <5 | nd |
| 17 | 1 | 2-NBA[g] | THF | 15 | 13 | 0 |
| 18 | 1 | TFA | THF | 5 | 60 | 44 |
| 19 | 1 | p-TSA | THF | 8 | 75 | 55 |
| 20 | 1 | CF3SO3H | THF | 6 | 71 | 20 |
| 21[h] | 1 | HCl | THF | 7 | 51 | 52 |
| 22[i] | 1 | HCl | THF | 5 | 73 | 61 |
| 23[i,j] | 1 | HCl | THF | 5 | 91 | 62 |
| 24[i,k] | 1 | HCl | THF | 5 | 97 | 64 |
| 25[i,k,l] | 1 [m] | HCl | THF | 6 | 81 | 73 |
| 26[i,k,l] | 1[n,o] | HCl | THF | 6 | 76 | 72 |
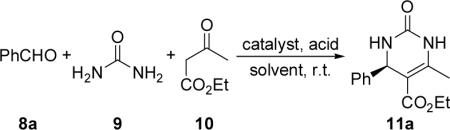
Unless otherwise noted, all reactions were conducted with compounds 8a (0.25 mmol), 9 (0.25 mmol), and 10 (0.25 mmol) in the presence of the catalyst (0.025 mmol, 10 mol %) and the acid cocatalyst (0.025 mmol, 10 mol %) in the specified solvent (1.5 mL) at room temperature.
Yield of the isolated product after column chromatography.
Determined by the HPLC analysis on a ChiralCel OD-H column.
The S-enantiomer was obtained as the major product.
Not determined.
2,2,2-Trifluoroethanol.
2-Nitrobenzoic acid.
Conducted with 5 mol % of the catalyst and 5 mol % of the acid cocatalyst.
Conducted with 20 mol % of the catalyst and 20 mol % of the acid cocatalyst.
Conducted with compounds 8a (0.25 mmol), 9 (0.375 mmol), and 10 (0.75 mmol).
Conducted with compounds 8a (0.25 mmol), 9 (0.50 mmol), and 10 (1.25 mmol).
The reaction temperature was 0 °C.
Catalyst 1 was recovered in 93% yield after the reaction.
Carried out with the recovered catalyst 1.
Catalyst 1 was recovered in 98% yield after the reaction.
