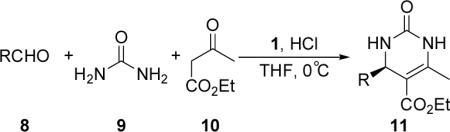
Table 2.
Three-component reaction of aldehydes, urea, and acetoacetate for the asymmetric synthesis of DHPMs[a]
| Entry | R | Product | Yield [%][b] | ee [%][c] |
|---|---|---|---|---|
| 1 | Ph | 11a | 81 | 73 |
| 2 | 4-MeC6H4 | 11b | 53 | 69 |
| 3 | 4-MeOC6H4 | 11c | 71 | 51 |
| 4 | 2-MeC6H4 | 11d | 53 | 53[d] |
| 5 | 4-FC6H4 | 11e | 61 | 73 |
| 6 | 4-ClC6H4 | 11f | 63 | 76 |
| 7 | 4-BrC6H4 | 11g | 68 | 78 |
| 8 | 4-CNC6H4 | 11h | 20 | 76 |
| 9 | 4-NO2C6H4 | 11i | 14 | 72 |
| 10 | 3-NO2C6H4 | 11j | 21 | 74 |
| 11 | n-C6H13 | 11k | 43 | 72 |
All reactions were conducted with compounds 8 (0.25 mmol), 9 (0.5 mmol), and 10 (1.25 mmol) in the presence of catalyst 1 (0.05 mmol, 20 mol %) and HCl (0.05 mmol, 20 mol %) in THF (1.5 mL) at 0 °C for 6 days.
Yield of the isolated product after column chromatography.
Unless otherwise noted, ee values were determined by the HPLC analysis on a ChiralCel OD-H column.
Determined by the HPLC analysis on a ChiralPak AD-H column.
