Abstract
Virtually all fields of physiological research now encompass various aspects of solute transport by convection, diffusion, and permeation across membranes. Accordingly, this set of terms, symbols, definitions, and units is proposed as a means of clear communication among workers in the physiological, engineering, and physical sciences. The goal is to provide a setting for quantitative descriptions of physiological transport phenomena.
Keywords: circulatory transport, diffusion, capillary permeability, flow, irreversible thermodynamics, tracer washout, pharmacokinetics
THE SET OF SYMBOLS is an extension of those proposed by Wood (11), Gonzalez-Fernandez(3), Zierler (12), Kedem and Katchalsky (5), and Bassingthwaighte et al. (1). The extensions provide a set of symbols common to studies of transcapillary and cellular exchange and indicator-dilution studies. The rationale is to provide a self-consistent set of symbols covering broad aspects of circulatory flows, hydrodynamics, transcapillary and membrane transport. As the various previously rather separate aspects of these fields become intermeshed, the size of the required sets of symbols has enlarged to a point where the “standard” symbol for one group of users has a quite different “natural” meaning to another. This problem has necessitated some arbitrariness, but we have attempted to subscribe to the dominant usage so as to minimize changes in habits.
Care has been taken to provide each term with 1) a name, 2) a definition in words (and sometimes equations), 3) a unique symbol whenever possible, and 4) units mainly in centimeter-gram-second system but with some translation to approved International System of units (SI). Physical constants are listed separately.
An important feature of this list is the provision of operational terminology for the general description of the behavior of linear stationary systems. The use of the time-domain impulse response or transport function, h(t), etc., follows from the work of Stephenson (10), Meier and Zierler (6), and Zierler (12) and is reviewed by Bassingthwaighte and Goresky (2).
A system is diagramed in Figure 1. Most analysis is based on two fundamental assumptions, that the system is both linear and stationary. When both hold, superposition is applicable. In general, we also consider the system to be mass conservative; that is, indicator and solvent are neither formed nor consumed.
FIG. 1.
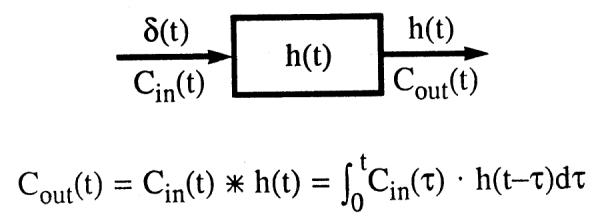
Block diagram of a linear stationary system. Response to ideal impulse input δ(t) at the entrance is h(t), the transport function. When input is of another form, Cin(t), then outflow response Cout(t) is the convolution of Cin(t) and h(t).
A linear system is one in which inputs and outputs are additive. Defining Cin(t), as concentration-time curve at the input to a segment of the circulation and Cout(t) as the concentration-time curve occurring in response to it at the outlet, the relationship is denoted by
Given a second pair with the same relationship , then in a linear system, these can be summed or multiplied by a scalar
A stationary system is one in which the distribution of transit times through the system is constant from moment to moment; that is, flows and volumes are constant everywhere in the system. Stationarity implies that the response to a given input is independent of a shift in the timing of the input by an arbitrary time, to,
When the input system is an ideal unit impulse, the Dirac delta function, δ(t), then the output is the transport function, h(t). When the input is of general form, Cin(t), and h(t) is known, then the form of the output, Cout(t), can be calculated using the convolution integral given in Fig. 1.
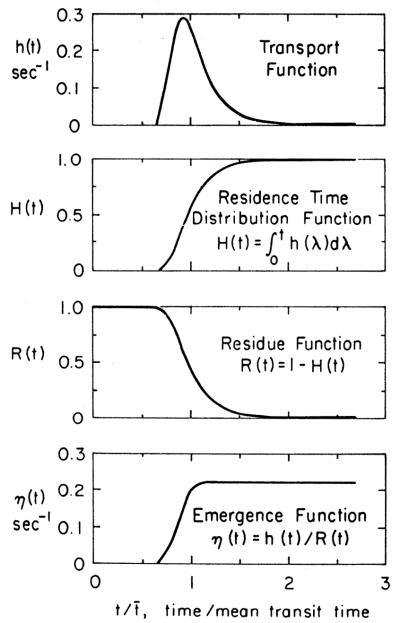
A probability density function h(x) or w(x) is a weighting function or a frequency function that gives the probability of occurrence of an observation or measure as a linear function of the quantitative measure, x. The sum of probabilities of all the observations is unity; therefore the units of the density function are fraction per unit of the measure [e.g., the transport function h(t)]. A typical form of h(t) for transport through an organ is given in Fig. 2, accompanied by closely related general functions.
FIG. 2.
Relationships between h(t), H(t), R(t), and η(t). Curve of h(t) is in this instance given by a unimodal density function having a relative dispersion of 0.33 and a skewness of 1.5. However, the theory is general and applies to h(t)s of all shapes. Tail of this h(t) curve becomes monoexponential and hence η(t) becomes constant.
Subscripts
| A | Arterial |
| B | Blood |
| C or cap | Capillary, or the region of blood-tissue ex- change |
| cell | Cell |
| D | Diffusive, or indicating a permeant tracer |
| ECF | Extracellular fluid |
| F | Flow or filtration |
| i,j | Indices in series or summations or elements of arrays |
| in or i | Into or inside or inflow |
| ISF or I | Interstitial fluid space, the extravascular extracellular fluid |
| m | Membrane |
| out or o | Out of or outside or outflow |
| P | Plasma |
| RBC | Red blood cell |
| R | Reference, nonpermeant tracer |
| S | Solute |
| T | Total |
| v | Venous |
| W | Water |
Principal Symbols
| a | Activity, molar; a = φC, an activity coefficient times a concentration |
|
| A | Area of indicator concentration-time curve excluding recirculation | |
| C | Concentration, mol/l; Cc(x, t) concentration in the capillary plasma at position x at time t (mol·1−1). Also [Na+] = sodium concentra- tion. The relationship between an outflow concentration-time curve Cout(t) and the inflow curve Cin(t) in a stationary system is given by the convolution integral: where τ is a variable used in the integration. The asterisk denotes convolution |
|
| C̄s | Concentration of solute, the average of the concentrations on the two sides of a mem- brane, molal, used in irreversible thermo- dynamic equations. Note that this average does not represent the mean concentration within the membrane when both convection and diffusion occur through a channel of finite length |
|
| CV | Coefficient of variation, dimensionless. See also RD; both are the standard deviation divided by the mean of a density function |
|
| D | Diffusion coefficient, cm2·s−1; Do, in free (aqueous) solution; Db for observed bulk diffusion coefficient through tissue; Dcell for intracellular; DI for interstitial |
|
| E | Electrical potential, volts; Em, membrane po- tential; EN, “Nernst” potential, occurring with a difference in concentration of an ion on the two sides of a membrane, EN = (RT/zF)loge(Cin,/Cout) |
|
| E(t) | Extraction, dimensionless, is the fraction of a specific substance removed during transit through an organ. The calculation may be made relative to a reference substance that remains in the blood or relative to the inflow concentration. E(t) = [hR(t) – ho(t)]/hR(t) and is the instantaneous apparent frac- tional extraction of a permeating species, subscripted D, relative to a nonpermeating reference substance, subscripted R, at each time t, calculated from paired outflow dilu- tion curves. This differs from a steady-state extraction, E, calculated from the arterio- venous difference, E = (CA – Cv)/CA, for a substance that is consumed during transor- gan passage. E(tpeak) is the value of E(t) obtained at the time of the peak of the curve for the nonpermeating reference tracer, hR(t). Emax is the maximum value of the instantaneous extraction, E(t). Enet(t) is an integral extraction, when the reference tracer has all emerged, then Enet(t) = RD(t), the retained fraction of a permeant solute |
|
| ECF | Extracellular fluid, interstitial fluid + plasma | |
| f | Frictional coefficient, g·cm equals (g·cm2 s−1)/(cm· s−1), following Spiegler (9) |
|
| fexcl | Excluded volume fraction, the fraction of sol- vent in a defined space that is not available to a particular solute, dimensionless |
|
| f i | Relative regional flow in the jth region of an organ divided by the mean flow for the organ per gram of tissue, dimensionless |
|
| F | Flow, cm3·s−1 or cm3·min−1 | |
| FB | Blood flow to an organ, cm3·g−1·min−1 (= F/W, where W = organ weight) |
|
| Fs, Fp | Flow of solute-containing mother fluid, cm3· g−1·min−1. When solute is excluded from red blood cells, Fs = FB(1 – Hct) = Fp, the plasma flow. (In modeling analysis, this is the flow of fluid containing solute available for exchange.) |
|
| FER(t) | Fractional escape rate at time t for indicator contained in a system regardless of time of entry, s−1. With an impulse input, δ(t), then FER(t) = η(t), the emergence function. In general, FER = (dq/dt)/q = d logeq/dt, where q is the system’s content of a sub- stance and dq/dt = F[Cin(t) – Cout(t)] |
|
| h(t) | Transport function, fraction/unit time (s−1), is the fraction of indicator injected at the inflow at t = 0, arriving at the outflow at time t. It is the unit impulse response, the frequency function of transit times, or the probability density function of transit times. The transport function, h(t), has the shape of the concentration-time curve that would be obtained by flow-proportional sampling at the output if indicator were injected in ideal fashion into the inflow, i.e., across a cross section with indicator amount at each point in proportion to local flow, as defined by Gonzalez-Fernandez (3), and re- circulation absent. Under such conditions h(t) = F·C(t)/qo, where qo is the mass injected at t = 0. Subscripting denotes re- gion (e.g., A, V, or cap) or solute character- istic (R for intravascular or D for permeant) |
|
| H(t) | Cumulative residence time distribution func- tion (dimensionless) of a system; it repre- sents the fraction of an ideally injected tracer that has exited from the system since t = 0. It is also the response to a step input. Formally, , where R(t) is the residue function |
|
| Hct | Hematocrit, the fraction of the blood volume that is erythrocytes, dimensionless |
|
| ISF | Interstitial fluid, the extravascular extracel- lular fluid |
|
| J | Flux, usually moles per unit surface area of membrane per second, mol·s−1·cm−2. Jnet 1→2 is net flux from side 1 to side 2. In the notation of irreversible thermody- namics the equations of Kedem and Katch- alsky (5) and Katchalsky and Curran (4) for water and solute transport across an ideal membrane composed of infinitely thin impermeant material pierced by aqueous channels (the K and K membrane) are where JD is a solute velocity relative to the solvent velocity, Jv, which is in turn relative to the membrane. [Although these expres- sions are incomplete in that the forces on the membrane, in effect a second solute, should also be considered (8), they provide an elementary conceptual approach to an idealized system.] Jv and JD may be properly regarded as flows rather than mass fluxes |
|
| J V | Solvent velocity or volume flux per unit mem- brane surface area relative to a membrane, cm·s−1 or cm3 · s−1 per cm2 area |
|
| J D | Solute movement relative to solvent, cm3 ·s−1 per cm2 surface area or cm·s−1. For the Kedem-Katchalsky (K-K) ideal membrane See JS |
|
| J W | Water flux across a membrane, mol·s−1·cm−2. For the K-K membrane |
|
| J S | Solute flux across a membrane, mol·s−1·cm−2. For the K-K membrane . Also |
|
| k | Rate constant for an exchange process, usu- ally s−1; k(C) is concentration-dependent rate |
|
| k F | Filtration coefficient, cm3·s−1·cmm−2 (mmHg)−1; kF = Lp. See Lp and also PF |
|
| K m | Michaelis constant, molar. For a reaction then Km = (k−1 + k2)/k1, which in the limit where k2 << k−1 becomes the original appar- ent dissociation constant, k−1/k1, which at equilibrium = [E]·[S]/[ES]. (E, enzyme; S, substrate; P, product) |
|
| l, L | Length, cm | |
| L | Conductance (general) per unit area as in J = LX; flux = conductance times driving force |
|
| L p | Pressure filtration coefficient or hydraulic conductance; the flow of pure solvent across a membrane per unit area per unit pressure difference, e.g., cm·s−1(mmHg)−1; also, |
|
| L pD | Osmotic coefficient; the flow of solution across a membrane per unit area per unit osmotic pressure difference. Same units as Lp; also, LpD = σLp |
|
| L Dp | Ultrafiltration coefficient; the conductance for the hydrostatically driven flow of solute relative to that of solvent, per unit area per unit hydrostatic pressure difference. Same units as Lp. By Onsager reciprocity, LDp = LpD. (For an ideal semipermeable mem- brane, σ = 1, ω = 0, and −LpD = Lp = LD = −LDp) |
|
| LD | Coefficient for diffusional mobility per unit osmotic pressure. Same units as LP. See ω and P |
|
| M | Molarity, moles of solute per liter of solution. Also mM, 10−3 M and μM, 10−6 M. (Molality is moles of solute per kilogram of solvent. The use of molal units gives consistency in transient states; for example, the molal con- centration of solute 1 is not changed by the removal of solute 2, but the molar concen- tration may be raised or lowered) |
|
| Mean |
, the mean of a density function, ω(x), is calculated by Same as α1 |
|
| ni | Same as α1 Moles of substance i in a solution. See mole fraction xi |
|
| N | Number of observations or number of ele- ments in a series, i = 1 to N |
|
| p | pressure, mmHg or Pa (1 Torr = 1 mmHg). See osmotic pressure, π |
|
| P | Permeability coefficient for a solute traversing a membrane, cm·s−1; equivalent to a diffu- sion coefficient for a solute in a membrane divided by the thickness. P = ωRT. P0, PL, permeabilities at the arterial and venous end of a capillary of length L, respectively. P(x) for 0 < x < L for permeability at position x. (Usually observed as a product, PS, with the membrane surface area, S) |
|
| Pe | Peclet number, ratio of a convective to a dif- fusive velocity, dimensionless |
|
| P F | Filtration permeability, , cm·s−1. [The conversion factor at 20°C, from the experimental units for Lp or kF, is (18.36 mmHg·cm3·mol−1)/( 18 cm3·mol−1) equals 1.02 mmHg] |
|
| PS | Permeability-surface area product of a barrier, cm3·g−1·s−1 or cm3·g−1·min−1. PScap for capillary (the same as capillary diffusion capacity), PScell for parenchymal cell |
|
| q | Mass, g or mol. q(t) is mass (or content of tracer) in region or organ (at time t). q0, mass of indicator injected at t = 0 |
|
| r, R | Radius or radial distance, cm. RC, capillary radius |
|
| RD |
. Same as coefficient of var- iation |
|
| R(t) | Residue function (dimensionless) is the com- plement of H(t), i.e., R(t) = 1 – H(t). It represents the fraction of injectate in the system at time t after an impulse input at time zero, i.e., the probability of a tracer residing in the system for time t or greater |
|
| S | Surface area. SC and Scell for capillary and cell surface areas, cm2·g tissue−1 |
|
| SD | Standard deviation = square root of the vari- ance of a density function, . Also (units are those of the independent variable) |
|
| SEM | Standard error of the , where N = number of observations |
|
| t, Δt | Time, s; Δt is a finite time interval | |
| Mean transit time, s. | ||
| t a | Appearance (a) time; the time at which the first detectable indicator (or a concentra- tion of, for example, 1% of the peak) passed through the system |
|
| t 0 | Zero time; midpoint of pulse injection for in- dicator-dilution studies or beginning of con- stant-rate injection |
|
| t peak | Time from injection to peak of indicator-di- lution curve (modal time) |
|
| V | Volume, cm3 or ml; in a solution, , the sum of the products of the mole fraction times the partial molar volume for each contained species |
|
| Vregion | Anatomic volumes within regions of an organ, i.e., VC, capillary; VI, interstitial fluid; Vcell, parenchymal cells, cm3·(g tissue of the organ)−1 |
|
| vregion | Fractional regional volumes of distribution available to a particular solute, i.e., vC, within the capillary; vI, interstitial fluid space; and vcell, parenchymal cells. At equi- librium, for a substance passively exchang- ing between plasma and ISF, vI is the ratio of the concentration in VI to that in the plasma and is equal to the partition co- efficient λ = CI/Cp. For steady-state proc- esses producing transmembrane fluxes, the effective volume of distribution is not the same as the equilibrium ratio, i.e., vI ≠ λ |
|
| ν F | Velocity of fluid flow, cm·s−1 | |
| V’ | Volumes of distribution, cm3·g−1. , in cap- illary; , in ISF; and , in parenchymal cell. These are the anatomic volumes times the fractional volume of distribution, e.g., . Commonly used ratios are and |
|
| Partial molar volume of solute i, cm3/mol; the increment in the volume of a solution per mole of added solute, e.g., |
||
| W | Mass, g (“weight,” mass times gravitational acceleration) |
|
| ω(x) | Weighting function or probability density function of variable x |
|
|
ωi or ωi(f) |
Weighting or fraction of total in the ith group. Units are fraction per unit of f. Given a density function of regional flows, ω(f), in its finite histogram representation ωiΔfi, is the fraction of the mass of the organ having a flow fi, the average of the flows grouped as the ith class. The fraction of the total flow going to the regions falling into the ith class is ωifiΔfi |
|
| x | Distance, cm; e.g., distance along the capillary from inflow, x = 0, to outflow, x = L |
|
| Mean of a density function, ω(x); see mean and moments, α |
||
| X | Generalized driving force | |
| xi | Mole fraction of component i; i.e., moles of the ith component divided by the total moles in the system, = ni/n, where n is the total |
|
| z | Valence of an ionic solute, number of unpaired electrons (or missing electrons) per molecule |
Greek symbols
| α0, α1, αn | Moments about zero for a probability density function. (Units are tn when t is the inde- pendent variable.) [α0 = area; α1 = mean; for the density function h(t), . See central moments, μ |
| β n–2 | Dimensionless parameters of shape of density function calculated from the central mo- ments, . “β1” is skewness (or asymmetry); β1 is zero for all symmet- rical functions, positive for right skewness. “β2” is kurtosis (or flatness). β2 = 3.0 for normal density function; β2 > 3 for lepto- kurtosis (highpeakedness), and <3 for platykurtosis |
| γ | Ratio of interstitial volume of distribution to intracapillary volume of distribution, |
| Δ | Difference |
| δ(t) | Unit impulse func- tion, or Dirac delta function, has unity area, an infinite amplitude at t = 0, and is zero at all other times. It is the limit of any symmetrical unimodal den- sity function of unity area as its width ap- proaches zero. For delta function occurring at a nonzero time t0, it is written δ(t – t0) |
| ∊ | Epsilon, vanishingly small difference |
| ζ | Tortuosity of diffusion pathway. ζ is ratio of apparent path length to measured length of diffusion pathway, dimensionless; thus the effective diffusion coefficient, D = D0/ζ2 where D is the free aqueous diffusion coef- ficient |
| η | Viscosity, poise (P) = dyn/cm2 = g·s−1·cm−1. Water viscosity = 0.01002 P at 20°C. Plasma viscosity ≈ 0.011 |
| η(t) | Equals h(t)/R(t) (fraction/s); the emergence function, the specific fractional escape rate following an impulse input. Of the particles residing in the system for t seconds after entering, η(t) is the fraction that will depart or escape in the tth second. In chemical engineering it is known as the intensity function (7), and in population statistics as the risk function, the death rate of those living at age t. Also, η(t) = (dR/dt)/R(t) = −d logeR(t)/dt. See FER(t) |
| Θ | Ratio of intracellular volume of distribution to intracapillary volume of distribution, , dimensionless |
| λ, λij | Partition coefficient, a dimensionless ratio of Bunsen solubility coefficients in two phases. λij is the ratio of solubility in region or solvent i to the solubility in region j. The reference region j is usually the plasma. At equilibrium, λij is the ratio of concentra- tions |
| μ | Chemical potential for a solute in a solution, N·m−2; μ = μ0 + RT In a, where the activity a is a concentration times an activity coef- ficient and μ0 is the potential at a reference state of temperature and pressure |
| μ n |
nth central moment of a density function, h(t), a moment around the mean, . . Units are those of t to the nth power |
| μ2, μ3, μ4 |
μ2 is variance, the second moment of a density function around the mean, . Also and . See also βn |
| π | Osmotic pressure, Pa or N·m−2 or mmHg, is the pressure that would have to be exerted on a solution to prevent pure water from entering it from across an ideal semiperme- able membrane, i.e., a membrane permeable to solvent only. π = CRT is Van’t Hoff’s law for ideal dilute solutions, and across a membrane impermeable to solute. π = φCRT is preferred to account for activity coefficients less than unity. When the sol- ute can permeate the membrane, the effec- tive π = σφCRT. Osmotic pressure, a colli- gative property of solutions, is related to actual pressure rn the same fashion as a freezing point is to actual temperature. On- cotic pressure is a term, now obsolete al- though historically useful, for the osmotic pressure associated with the presence of large, relatively impermeant molecules such as plasma proteins. It should now be re- placed by more exact terms, e.g., across some specific membrane the effective Δπ equals , where the effects of concentration differences for a set of N solutes are summed. |
| ρ | Density, g·cmm−3. (Specific gravity is density relative to density of water) |
| σ | Reflection coefficient, in notation of irrevers- ible thermodynamics, dimensionless; σ = –LpD/Lp or, experimentally, σ = –JD/Jv for ΔC8 = 0. The effective osmotic pressure across a membrane is σΔπ, mmHg; i.e., σ = (observed osmotic pressure)/CRT |
| τC | Capillary mean transit time, t̄c, used in Krogh cylinder capillary-tissue models with plug flow velocity profiles |
| φ | Activity coefficient, the ratio of apparent chemically effective concentration to the actual concentration in a solution, in the absence of chemical binding, dimensionless. The osmotic activity coefficient φ = π/CRT |
| ψ | Electrical potential, V |
| ω | Solute permeability coefficient, ω = P/RT, mol·cm−2. s−1 ·(mmHg)−1. In the notation of irreversible thermodynamics ω = (LD – RT/F σ2Lp)C̄s, where C̄s, is the average solute con- centration across the membrane |
Physical Units, Constants
| A | Ampere, unit of electrical current, coulomb per second (C·s−1) |
| Å | Ångstrom, 10−10 m or 0.l nm |
| C | Charge, coulomb, ampere· second (A. s) |
| °K | Degrees of temperature, Kelvin (absolute); °C for degrees Celsius = 273.15 + °K |
| dyn | Dyne, force, g·cm·s−2 = 10−5 N (newton) |
| eq | Equivalent weight = molecular weight/va- lence. One equivalent carries 9.65 × 104 C of charge |
| e | Elementary charge, 1.6021892 × 10−19 C |
| erg | Energy, dyn·cm = g·cm2·s−2 = 10−7 J |
| F | Faraday constant, 9.648456 × 104 elementary charge. eq−1 = 96,484.6 C· mol−1 = NAe |
| g | Acceleration due to gravity = 980.665 cm·s−2 |
| h | Planck’s constant (energy quantum) = 6.626176 × 10−27 erg·s = 6.626 × 10−34 J·s |
| η | Viscosity; 1 poise (P) = 1 cm−1· g·s−1 = 0.1 Pascal·second (Pa·s) |
| I | Current, amperes |
| J | Joule = Watt·second (W ·s) = ampere·volt· second (A·V·s) = 107 erg = 107 cm2g·s−2 |
| k | Boltzmann constant, 1.380662 × 10−23 J. °K−1 = R/NA, the gas constant over Avogadro’s number = 1.37900 × 10−16 cm2·g·s−2·°K−1 |
| 1, liter | Liter = 1 dm3 = 1,000 cm3. Also milliliter (ml) and microliter (μl) |
| M | Mol/l (molarity) |
| mol/kg | Mol solute/kg solvent (molality) |
| N | Newton = 105 dyn = 105 cm·g·s−2 |
| N A | Avogadro’s number, 6.022045 × 1023 mol−1, the number of molecules contained in 1 mol |
| ns, nw | Number of moles of solute and water |
| P | Pressure (= force per unit area), N·m−2 or Pa (Pascal). (1 Pa ≡ 1 N·m−2 ≡ 10 g·cm−1·s−2 ≡ 10−2 mbar ≡ 0.10197 mmH20 ≡ 7.5 × 10−3 mmHg ≡ 9.869 × 10−6 atm; or 1 atm = 101325 Pa = 760 Torr; 1 cmH20 (at density 1 g·cm−3) = 98.0665 Pa = 981 g·cm−1·s−2; 1 mmHg = 1.00000014 Torr = 133.322 Pa = 1,333 g·cm−1 · s−2 |
| ρ | Density, g·cm−3. Water (3.98°C, 1 atm) = 0.999972 g·cm−3. Mercury (0°C, 1 atm) = 13.59508 g·c−3 |
| R | Resistance, electrical (Ω), or electrophysiolog- ical (Ω/cm2) or vascular (a pressure divided by a flow) |
| R | Universal gas constant = 8.31441 J.mol−1 °K−1 = 8.3144 × 107 cm2 ·g·s−2 ·mol−1 °K−1 = 0.082 1· atm· mol−1 °K−1 = 0.0623 mmHg· mmol−1· °K−1 = 8.31441 × 10−7 erg·mol−1· °K−1 |
| RT | Energy/mol, gas constant × absolute temper- ature; e.g., at 37°C or 310.16°K, RT = 19.34 × 106 mmHg· cm3· mol−1 |
| RT/F | 24.84 mV at 15°C.26.62 mV at 37°C. Values of loge,10 RT/F at 15, 20, 25, 30, and 37°C are 57.2, 58.2, 59.2, 60.2, and 61.3 mV |
| STP | Standard temperature and pressure (ice point of water, 0°C = 273.16°K; 760 mmHg = 1 atm = 1.01325 × 106 dyn·cm−2 = 1.013 × 105 N· m−2) |
| T | Temperature, absolute, in degrees Kelvin (°K); 0°C = 273.16 °K |
| V | Volts; millivolt, mV; microvolt, μV |
| Ṽ i | Partial molar volume, ml/m01 = (∂V/∂ni)T,p,njj≠i = change of volume of total system per mole additional solute i, at T, p, and constant presence of other components j, and at the particular concentration ni/V. (ṼW is the partial molar volume of water; close to 18 ml/mol for physiological solu- tions) |
| Watt | Unit of power, joules per second, J·s−1 |
| Work | Work is energy × time or force × distance × time, erg·s or J· s or cm2g·s−1 |
| Ω | Ohm, unit of electrical resistance; V/I |
Acknowledgments
The authors greatly appreciate the efforts of Geraldine Crooker in the preparation of this terminology.
REFERENCES
- 1.Bassingthwaighte JB, Chinard FP, Crone C, Lassen NA, Perl W. Definitions and terminology for indicator dilution methods. In: Crone C, Lassen NA, editors. Capillary Permeability. Munksgaard; Copenhagen: 1970. pp. 665–669. [Google Scholar]
- 2.Bassingthwaighte JB, Goresky CA. Handbook of Physiology. Sect. 2, The Cardiovascular System. Vol. IV, The Microcirculation. Bethesda, MD: Modeling in the analysis of solute and water exchange in the microvasculature. [Google Scholar]; Am. Physiol. Sot. 1984:549–626. chapt. 13. [Google Scholar]
- 3.Gonzalez-Fernandez JM. Theory of the measurement of the dispersion of an indicator in indicator-dilution studies. Circ. Res. 1962;10:409–428. doi: 10.1161/01.res.10.3.409. [DOI] [PubMed] [Google Scholar]
- 4.Katchalsky A, Curran PF. Nonequilibrium Thermody-namics in Biophysics. Harvard Univ. Press; Cambridge, MA: 1965. [Google Scholar]
- 5.Kedem O, Katchalsky A. Thermodynamic analysis of the permeability of biological membranes to non-electrolytes. Biochim. Biophys. Acta. 1958;27:229–246. doi: 10.1016/0006-3002(58)90330-5. [DOI] [PubMed] [Google Scholar]
- 6.Meier P, Zierler KL. On the theory of the indicator-dilution method for measurement of blood flow and volume. J. Appl. Physiol. 1954;6:731–744. doi: 10.1152/jappl.1954.6.12.731. [DOI] [PubMed] [Google Scholar]
- 7.Shinnar R, Naor P. Residence time distributions in systems with internal reflux. Chem. Eng. Sci. 1967;22:1369–1381. [Google Scholar]
- 8.Silberberg A. The mechanics and thermodynamics of separation flow through porous, molecularly disperse, solid media—the Poiseuille Lecture 1981. Biorheology. 1982;19:111–127. doi: 10.3233/bir-1982-191-214. [DOI] [PubMed] [Google Scholar]
- 9.Spiegler KS. Transport process in ionic membranes. Trans. Faraday Sot. 1958;54:1408–1428. [Google Scholar]
- 10.Stephenson JL. Theory of the measurement of blood flow by the dilution of an indicator. Bull. Math. Biophys. 1948;10:117–121. doi: 10.1007/BF02477486. [DOI] [PubMed] [Google Scholar]
- 11.WOOD EH. Definitions and symbols for terms commonly used in relation to indicator-dilution curves. Circ. Res. 1962;10:379–380. [Google Scholar]
- 12.Zierler KL. Equations for measuring blood flow by external monitoring of radioisotopes. Circ. Res. 1965;16:309–321. doi: 10.1161/01.res.16.4.309. [DOI] [PubMed] [Google Scholar]