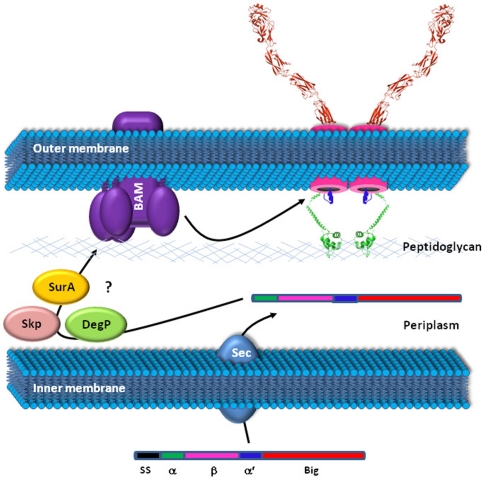
Figure 8. Proposed model for Intimin/Invasin biogenesis.
Int/Inv proteins are synthesized as single polypeptides possessing a modular organization consisting of a signal peptide (SS), an α-hydrophilic domain (α), an N-proximal β-domain (β), a second α-helical domain (α′) and a C-terminal passenger domain (Big) which adopts an immunoglobulin-like fold. The signal peptide mediates translocation of the Int/Inv protein across the inner membrane by a post-translational Sec-dependent mechanism. Once periplasmically located, the signal peptide is removed, releasing the remainder of the molecule into the periplasm as an intermediate. The periplasmic intermediate is bound by periplasmic chaperones such as SurA, Skp and DegP and delivered to the β-barrel assembly apparatus (the BAM complex: TC# 1.B.33). The BAM complex facilitates folding of β into a β-barrel structure and insertion of the barrel into the outer membrane, where β adopts a homodimeric conformation. During this process, α remains periplasmically located, and those proteins with a LysM domain interact directly with the peptidoglycan. Such interactions may alter the porosity of the peptidoglycan to allow the bulky disulphide bonded portion of the passenger domain to migrate to the pore formed by β. Once β is inserted correctly into the outer membrane, Big is translocated to the cell surface. It remains unclear whether Big adopts the immunoglobulin-like fold before or after the translocation event. The α′ domain may be inserted into the pore formed by β in a manner analogous to the autotransporters, facilitating translocation of Big to the cell surface and sealing the pore after the translocation event.