Fig. 3.
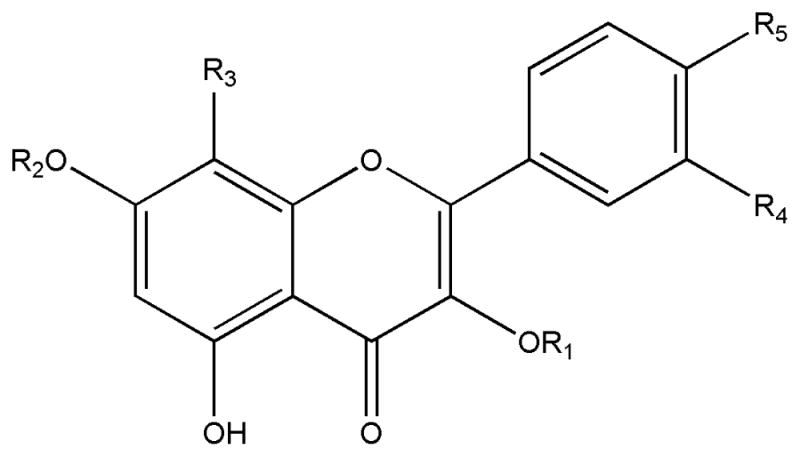
Chemical structures of ten flavonols from Flos Gossypii isolated by HSCCC
| No. | compound | R1 | R2 | R3 | R4 | R5 |
| I | Kaempferol-3-O-β-D-(6″-O-p-coumaroyl)-glucoside | (6″-p-coumaroyl) –Glu | H | H | OH | OH |
| II | 8-methoxyl-kaempferol-7-O-β-D-rhamnoside | H | Rha | OMe | H | H |
| III | Astragalin | Glu | H | H | H | H |
| IV | 4′-methoxyl-quercetin-7-O-β-D-glucoside | H | Glu | H | OH | OMe |
| V | Quercetin-3′-O-β-D-glucoside | H | H | H | OGlu | H |
| VI | -- | -- | -- | -- | -- | -- |
| VII | Hyperoside | Gal | H | H | OH | H |
| VIII | Quercetin-3-O-β-D-glucoside | Glu | H | H | OH | H |
| IX | Quercetin-7-O-β-D-glucoside | H | Glu | H | OH | H |
| X | Quercetin | H | H | H | OH | H |
