Figure 3.
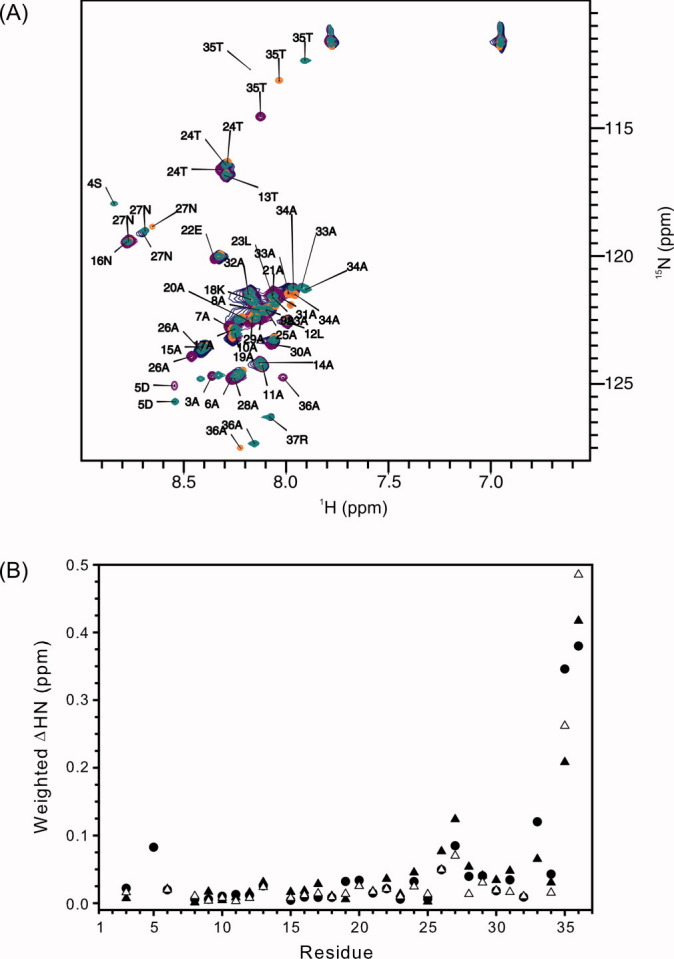
Local structure of rHPLC6, rHPLC6-Ala37-NH2, rHPLC6-Arg37, and rHPLC6-Ala37. (A) Overlapped 15N-HSQC spectra of rHPLC6 and mutants at 3°C. The residues of all of the assigned peaks of the wild-type protein are labeled with the residue number and single-letter amino acid code. Peaks of the mutant proteins that have shifted significantly [>0.05 ppm as shown in (B)] are also labeled. rHPLC6, purple; rHPLC6-Ala37-NH2, blue; rHPLC6-Arg37, green; rHPLC6-Ala37, orange. (B) Chemical shift changes of the mutants compared with the wild-type protein. Weighted changes in backbone 1H and 15N chemical shifts of rHPLC6-Ala37-NH2 (open triangles), rHPCL6-Arg37 (solid circles), and rHPLC6-Ala37 (solid triangles) are plotted on a per residue basis.
