Figure 4.
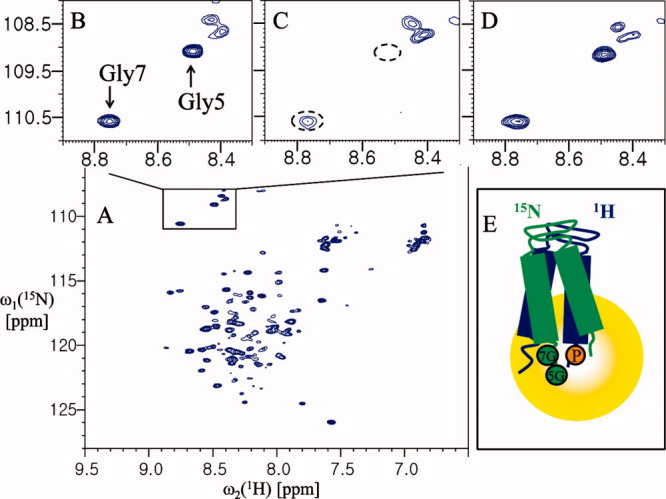
Analysis of the interprotomer interactions of YbdK-TM. (A) The 2D 1H-15N HSQC spectrum of 0.4 mM YbdK-TM at 40°C in 20 mM sodium acetate pH 4.8 and ∼70 mM DPC. (B) The 1H-15N correlation peaks of Gly5 and Gly7. (C) The 1H-15N correlation peaks of the glycine residues of the protein (0.4 mM) in the presence of 1.2 mM spin-labeled YbdK-TM (Ser73Cys) with nitrogen at natural abundance. (D) The same sample as for (C) but after incubation with 20 mM DTT for 2 h at 37°C. (E) Model of dimer topology of YbdK-TM in DPC micelle. The 5G and 7G (in the green circles), and P (in the yellow circle) represent residues Gly5 and Gly7, and the paramagnetic probe at Cys73 position, respectively. The peak intensities of Gly7 were measured relative to a reference peak that was not affected by paramagnetic perturbation (at 8.11 and 112.82 ppm for the 1HN and 15N chemical shifts, respectively). The resulting values were as follows: B, 3.74; C, 1.14; and D, 2.74.
