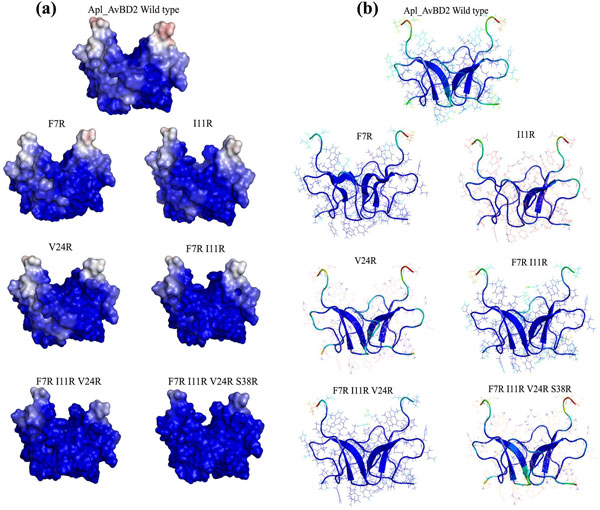
Figure 3.
Distribution of charged residues and flexibility of Apl_AvBD2 and its mutants during MD simulations. (a) Distribution of charged residues on the solvent-accessible surfaces. Positively charged residues are represented as blue and negatively charged areas shown as red. (The potentials range from -5 kT/e for red to +5 kT/e for blue). As the number of arginine residues increased the cationicity of the peptide surface also increased. (b) Flexible regions in the average MD simulation structure of the peptides. The C-terminal residues and the loops of wild type, F7R, I11R and F7R I11R V24R S38R appeared to be more flexible. The dark blue areas represent the rigid regions and flexibility of the structure increases as dark blue turns light blue to red. The length of the β-sheets was varied in some of the mutants. The single amino acid mutant of Apl_AvBD2 (I11R) showed loss of two β-sheets in its structure.