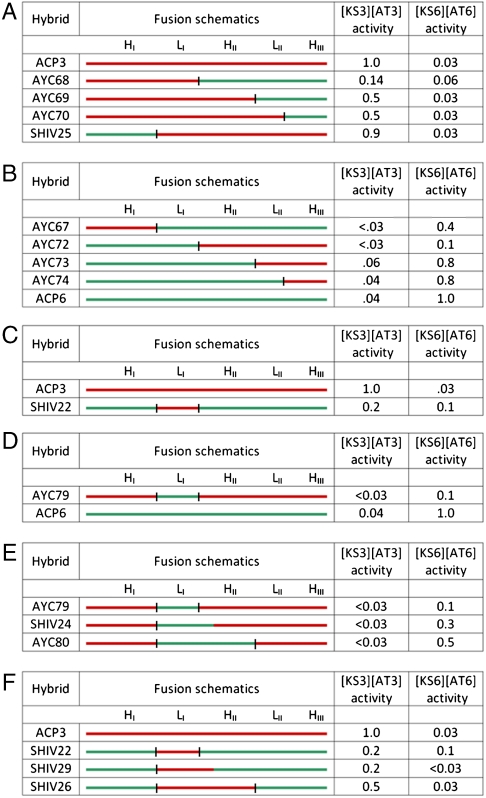
Fig. 3.
Evaluation of chimeric ACP proteins in an assay for polyketide chain elongation. A series of chimeric ACP proteins were constructed and assayed with homodimeric [KS][AT] proteins harboring either KS3 or KS6. For each chimera the activity with [KS3][AT3] or [KS6][AT6] is normalized to the corresponding wild-type ACP. The initial set of chimeras are shown in panels A and B. (A) ACPs that prefer [KS3][AT3] over [KS][AT6]. (B) ACPs that prefer [KS6][AT6] over [KS3][AT3]. Because these preferences tracked with the identity of LI, SHIV22 (C) and AYC79 (D) were constructed and found to have the predicted [KS][AT] preference, albeit at the expense of reduced activity. Additional chimeras, shown in Panels E and F, were engineered to further optimize activity and specificity. Specifically, redefinition of the LI-HII junction of SHIV22 and AYC79 afforded SHIV24 (E) and SHIV29 (F), respectively. The color scheme is similar to Fig. 2 (red = ACP3 derived, green = ACP6 derived). Fusion sites (black bars) are consistent with Fig. 2. For SHIV24 and SHIV29 the C-terminus of the substituted fragment is at residue 58. SHIV22, 25, 26 and 29 harbor a H26A mutation (see SI Text for details).