Fig. 4.
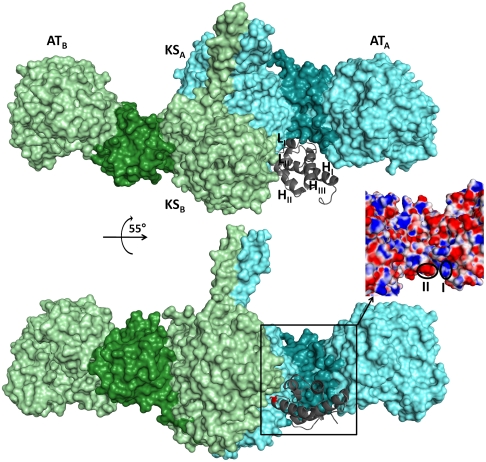
Docking model for ACP5 domain with the homodimeric [KS5][AT5] protein. Although the ACP domain of monomer A (gray) occupies a deep cleft between the KS and AT domains of monomer A (light cyan) (A), the conserved serine (red sticks) (B) at the N-terminal end of HII is positioned to participate in polyketide chain elongation with the KS active site of monomer B (light green). The KS-AT linker region of each monomer, which interacts with LI of the ACP domain (gray), is highlighted (dark cyan or dark green). The inset shows the two regions that show electrostatic complementarity with residue 44 (region I) and residue 45 (region II).