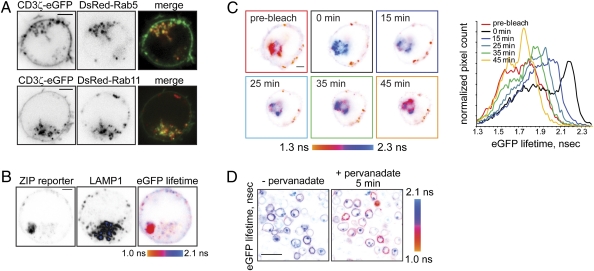
Fig. 4.
Lck locally phosphorylates CD3ζ at the plasma membrane and on endosomes. (A) Jurkat E6.1 cells, transiently coexpressing CD3ζ-eGFP and either DsRed-tagged Rab5 (Upper) or Rab11 (Lower), were adhered onto a poly-d-lysine–coated glass-bottom dish. Shown are the confocal images in the eGFP or DsRed channels and the confocal overlay at the midcell level, so that intracellular vesicles are clearly seen. Time-lapse sequences are presented in Movies S4 and S5. (Scale bars, 2 μm.) (B) ZIPWT reporter-expressing Jurkat cell was fixed and immunostained using mouse LAMP1 antibody and secondary goat anti-mouse Alexa647-conjugated Ab. Shown are the confocal images of the reporter in the eGFP channel and antibody in the far-red channel, as well as the corresponding eGFP lifetime image, scaled between 1.0 and 2.1 ns. Internalized tyrosine-phosphorylated reporter is present in intracellular vesicles distinct from lysosomes. Low-background signal colocalizing with the LAMP1 staining likely indicates degradation of the reporter in the lysosomes. (Scale bar, 2 μm.) (C) Jurkat cell transiently expressing the ZIPWT reporter was adhered onto a glass-bottom dish, precoated with 10 μg/mL mAb HIT3a, and eGFP lifetime before and after acceptor (mCherry) photobleaching was monitored. Shown are pseudocolored eGFP lifetime images, scaled between 1.3 and 2.3 ns. mCherry fluorescence recovery is shown in Fig. S4F. Right: Corresponding cumulative eGFP lifetime histograms. (Scale bar, 2 μm.) (D) Lck-deficient J.CaM1 cells stably expressing the ZIPWT reporter were adhered onto a poly-d-lysine–coated glass-bottom dish for 10 min. eGFP fluorescence lifetime was monitored using TCSPC-FLIM before and 5 min after addition of 1 mM sodium pervanadate. In this Lck-deficient cell line, 5 min stimulation with pervanadate results in a FRET increase at the plasma membrane but not in the intracellular vesicles. (Scale bar, 20 μm.)